When a system at equilibrium contains at least one gaseous substance, a change in pressure can alter its equilibrium. This is because a variation in pressure changes the volume in which a certain quantity of gas molecules is contained. Based on Le Chatelier's principle, the consequences of a change in pressure can be summarised as follows:
When the pressure of a reaction system is modified, the reaction at equilibrium tends to oppose the modification.
-
If the pressure increases, the system will react by favouring the direction of reaction that produces the smallest number of gas molecules in order to restore the pressure.
-
If the pressure decreases, the system will react by favouring the direction of reaction that produces the greatest number of gas molecules in order to restore pressure.
To understand the effect of a variation in pressure on the state of equilibrium, let's take the following example:
|N_{2(g)} + 3\; H_{2(g)} \rightleftharpoons 2\; NH_{3(g)}|
in which there are 4 moles of gaseous reactants for every 2 moles of gaseous products.
When we want to determine the influence of pressure on the equilibrium state, we consider only the volumes occupied by the gases.
The volume occupied by a liquid, a solid or a system of ions in an aqueous medium is not influenced by a variation in pressure because these substances are not compressible fluids. They are therefore assigned an arbitrary value of 0 volume.
The pressure of a reaction mixture is changed by changing the volume of the container in which the reaction takes place. The pressure and volume of a gas are inversely proportional. An increase in volume leads to a decrease in pressure, while pressure increases when volume decreases.
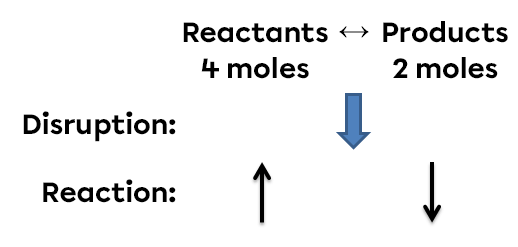
According to Le Chatelier's principle, a system in equilibrium reacts in such a way as to partly oppose the changes imposed on it. Thus, following an increase in pressure, the reaction system will react in the opposite way, favouring the direction of the reaction that reduces the number of gaseous molecules.
In this example, an increase in pressure creates an imbalance. The gas molecules are compressed. To return to a new state of equilibrium, the system opposes itself and favours the reaction that reduces the pressure. In this way, the side of the reaction containing the lowest number of moles (the lowest coefficients) will be favoured in order to reduce the pressure. In this situation, the quantity of reactants will decrease while the quantity of products will increase.
To sum up, if we increase the pressure in this system, the equilibrium will shift to the right. It is on the right that there are the fewest moles of gas. The forward reaction is therefore favoured. There will then be fewer reactants, but more products in the new state of equilibrium.
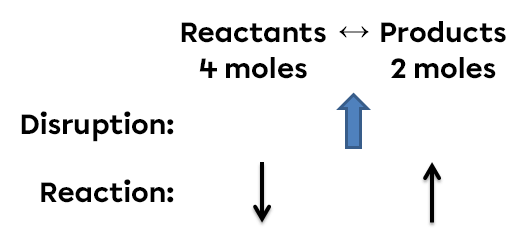
According to Le Chatelier's principle, a system in equilibrium reacts in such a way as to partly oppose the changes imposed on it. Thus, following a decrease in pressure, the reaction system will react in the opposite way, favouring the direction of the reaction that increases the number of gaseous molecules.
In this example, a decrease in pressure creates an imbalance. The gaseous molecules are spaced apart. To return to a new state of equilibrium, the system opposes this change and favours the reaction that increases the pressure. Thus, the side of the reaction containing the greatest number of moles (the highest coefficients) will be favoured in order to increase the pressure. In this situation, the quantity of reactants will increase while the quantity of products will decrease.
To sum up, if we reduce the pressure in this system, the equilibrium will shift to the left. It is on the left that there are the most moles of gas. This favours the reverse reaction. There will then be more reactants, but fewer products in the new state of equilibrium.