This concept sheet explains the steps for identifying the nutrients in food.
There are several tests that can identify the nutrients in various foods. The protocol for each of these tests is explained below.
| Indicator | Nutrient |
| Fehling's solution | Carbohydrates |
| Lugol’s iodine | Starch |
| Sudan IV | Lipids |
| Biuret reagent | Proteins |
| Indophenol | Vitamin C |
| Silver nitrate | Chlorine in mineral salts |
| Ammonium oxalate | Calcium |
To perform these tests, it is essential that the food is in liquid form. If the food is not liquid, it must be crushed and mixed with distilled water, if necessary, to help liquidize the food.
-
Test tube
-
Test tube rack
-
Test tube clamp
-
Hot plate
-
400 mL beaker
-
Water
-
Dropper
-
Food in liquid form
-
Fehling's A and Fehling’s B
-
Apron or lab coat
-
Safety glasses or goggles

-
Fill the beaker up halfway with tap water.

-
Place the beaker on the hot plate and heat to boiling.

-
Measure 20 drops of the liquid food and deposit them into a test tube.

-
Measure 10 drops of Fehling's A and add them into the test tube.

-
Measure 10 drops of Fehling's B and add them into the test tube.

Fehling's B is a corrosive substance. Avoid any contact with your hands or any other part of the skin.
-
Place the test tube in the beaker filled with boiling water and leave it in the water for one minute.

It is best to use the test tube clamp to put in or remove the test tube from the beaker to avoid splashing boiling water on your hands.
-
Take the test tube out of the boiling water and observe the colour of the solution.

-
Clean and store the equipment.
Depending on the contents of the food, there are two possible results.
-
If Fehling's solution does not change colour and stays blue, this indicates that there are no monosaccharides (like glucose) or disaccharides (like lactose) in the food.

-
If Fehling's solution turns orange-brown, this indicates the food contains monosaccharides (like glucose) or disaccharides (like lactose).

-
Test tube
-
Test tube rack
-
Food in liquid form
-
Lugol’s iodine
-
Dropper
-
Apron or lab coat
-
Safety glasses or goggles

-
Place the food to be tested in the test tube.

-
Take a few drops of Lugol’s iodine and add them into the test tube.

-
Observe the colour obtained.

-
Clean and store the equipment.
There are two possible outcomes depending on the contents of the food.
-
If the Lugol’s iodine stays yellow, there is no starch in the food.

-
However, if the food turns purple-black after Lugol’s iodine is added, this indicates that the food contains starch.

There may be a brown colour after mixing the Lugol’s iodine with the food. Such a colour is produced when glycogen, a long chain of sugars formed with glucose, is present in the food.

-
Test tube
-
Test tube rack
-
Rubber stopper
-
Dropper
-
Food in liquid form
-
Sudan IV
-
Spatula
-
Apron or lab coat
-
Safety glasses or goggles
-
Gloves

-
Measure 20 drops of the food and put them into a test tube.

-
Put a few drops of Sudan IV into the test tube.

-
Close the test tube with a stopper.

-
Shake the test tube lightly.
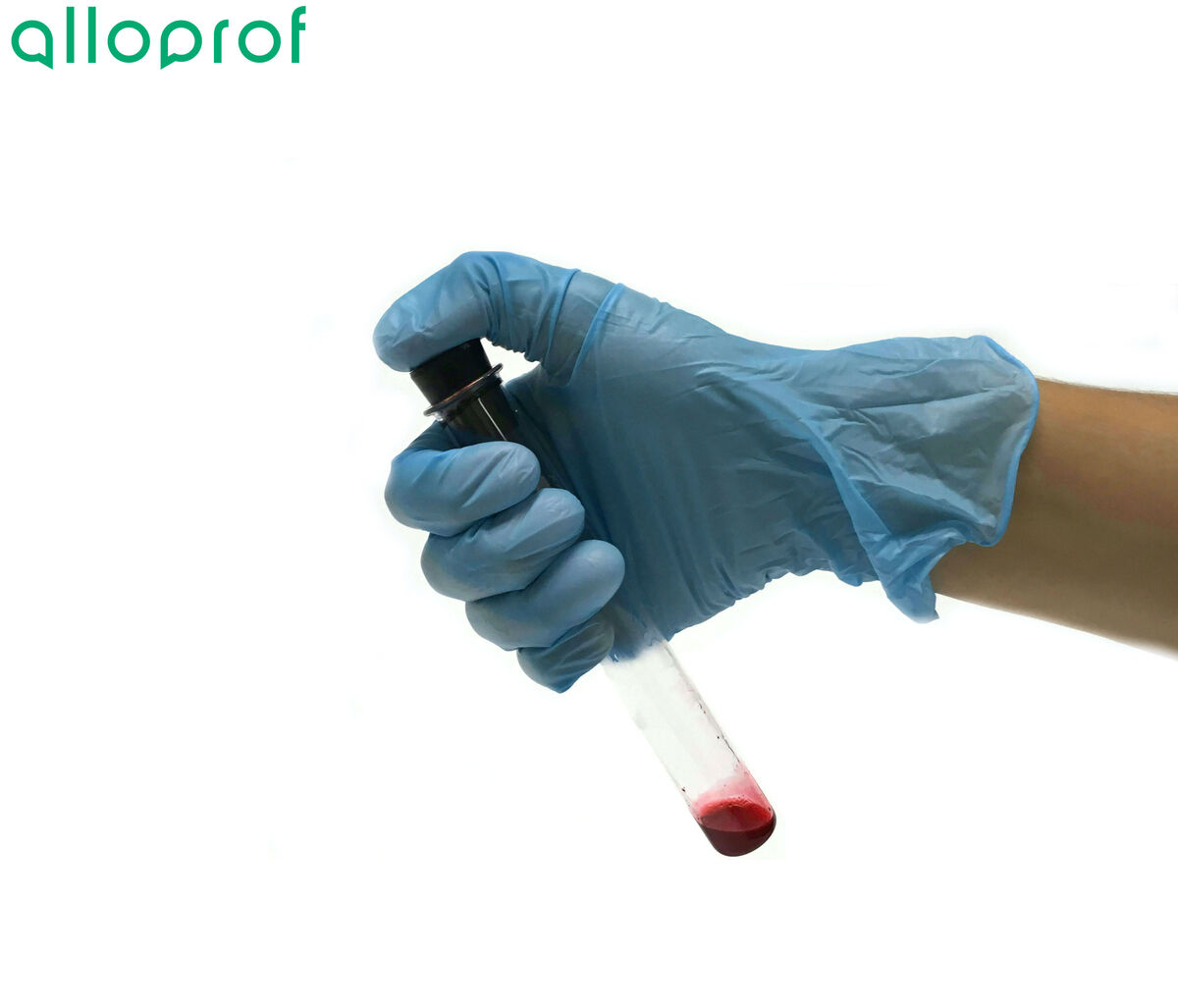
To prevent the mixture from spilling, hold the test tube by the upper part and keep your thumb on the stopper. This will ensure the stopper seals better on the test tube.

-
Observe the colour obtained.

-
Clean and store the equipment.
Two results are possible depending on the contents of the food.
-
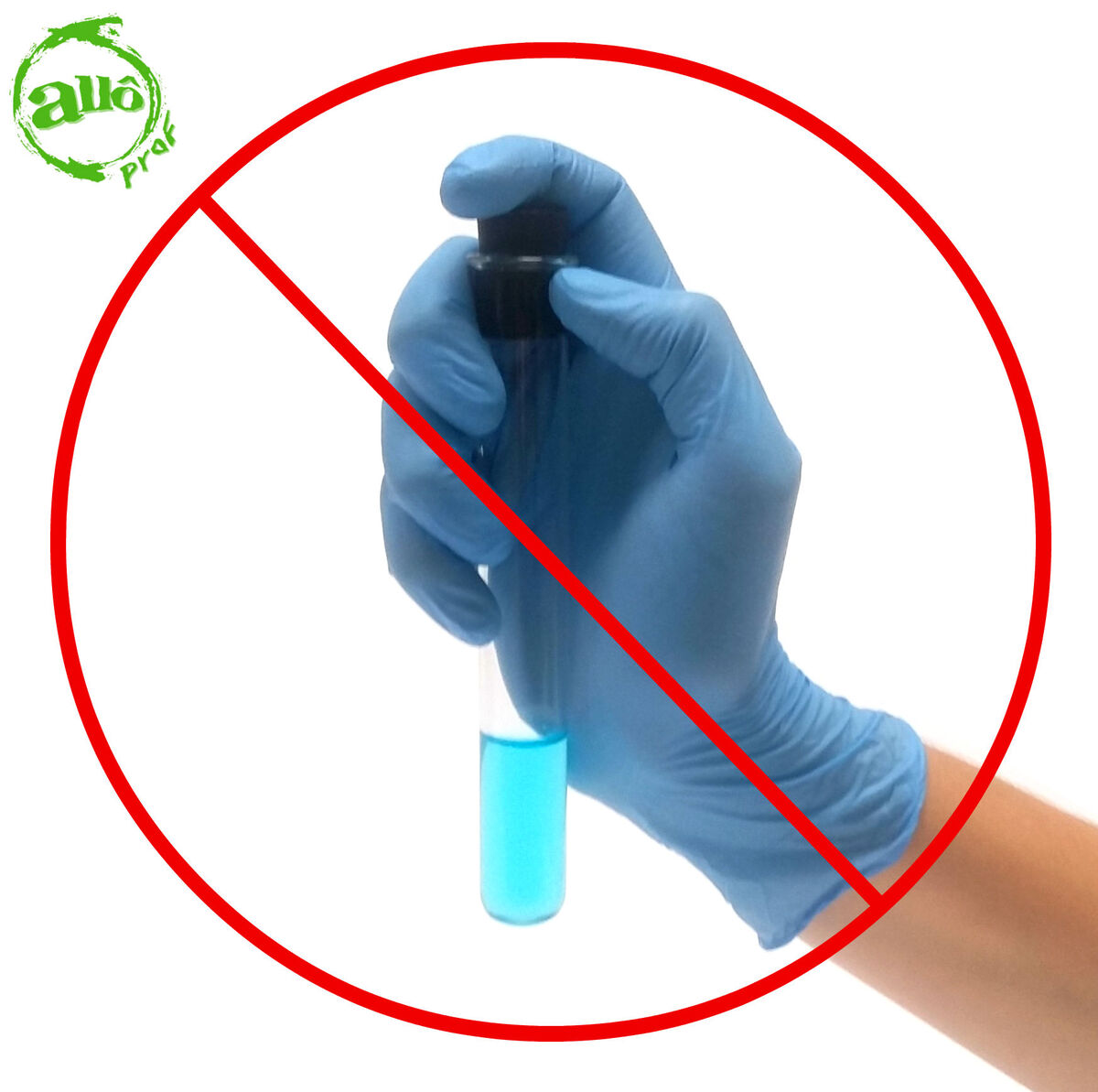
If the Sudan IV does not dissolve in the solution, the food does not contain lipids.
-
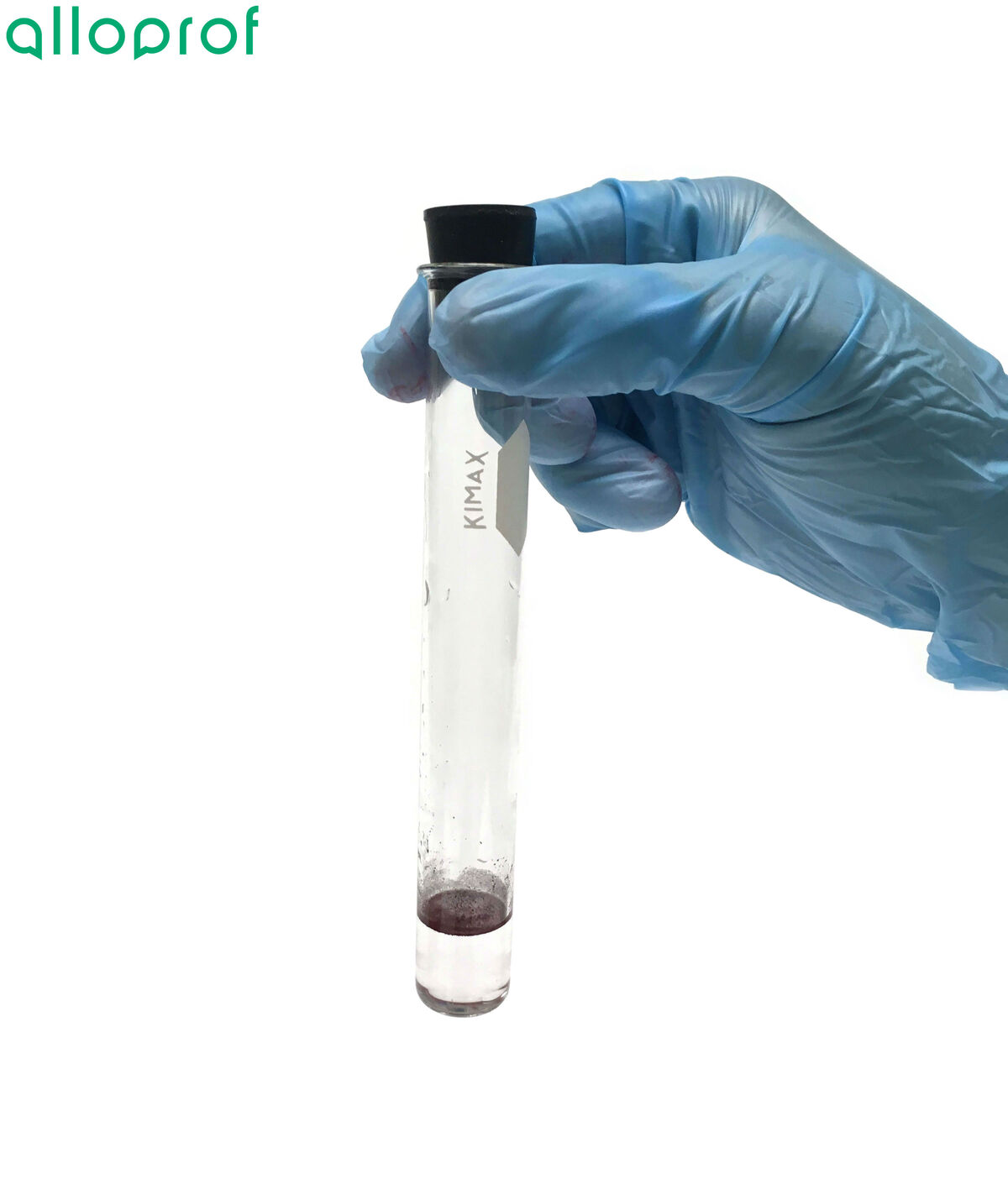
If the Sudan IV dissolves and produces a red colour, the food contains lipids.
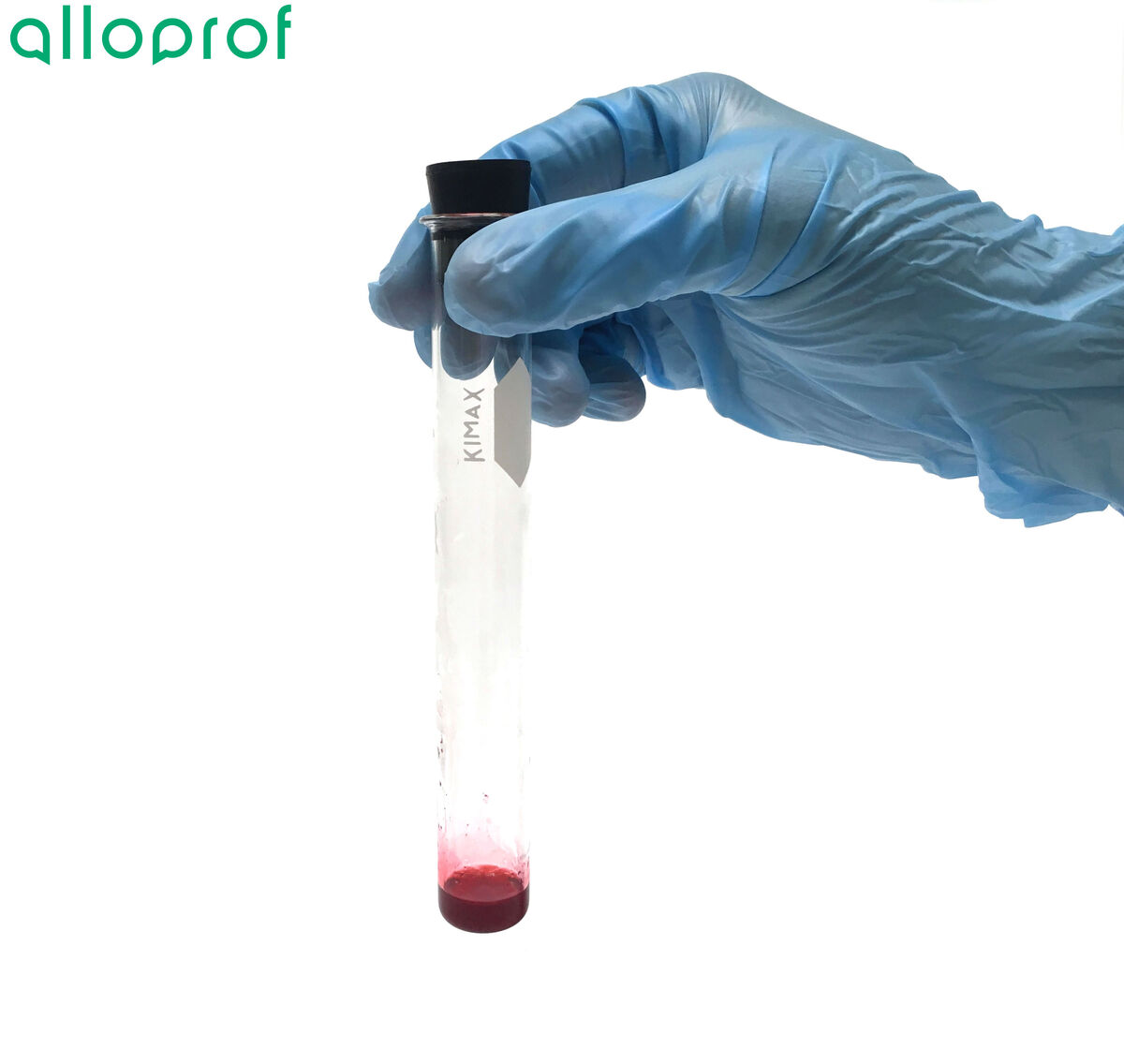
If the food is solid, it is also possible to check for the presence of lipids using the paper test. Simply rub the substance to be tested on a piece of paper and then allow the paper to dry. The presence of lipids is indicated by transparent circles on the surface.

-
Test tube
-
Test tube rack
-
Dropper
-
Food in liquid form
-
Biuret reagent
-
Apron or lab coat
-
Safety glasses or goggles

-
Measure 20 drops of the food and put them into a test tube.

-
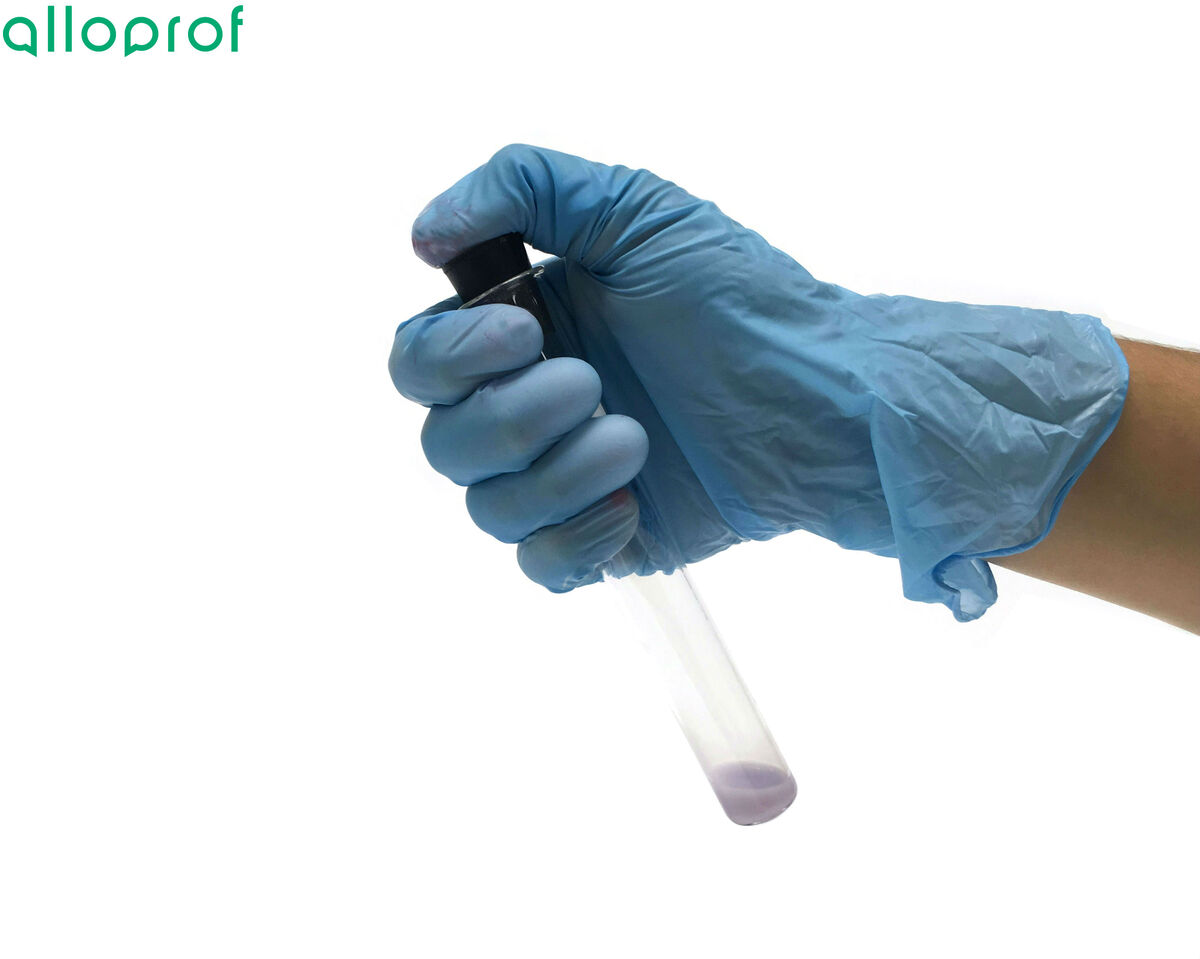
Squeeze seven drops of the Biuret reagent into the test tube.

-
Shake the test tube.
-
Observe the colour obtained.

-
Clean and store the equipment.
The colour obtained indicates the presence or absence of proteins.
-
If the solution is a light blue colour, the food does not contain any proteins.

-
If the solution is a purple colour, the food contains proteins.

-
Test tube
-
Test tube rack
-
Dropper
-
Food in liquid form
-
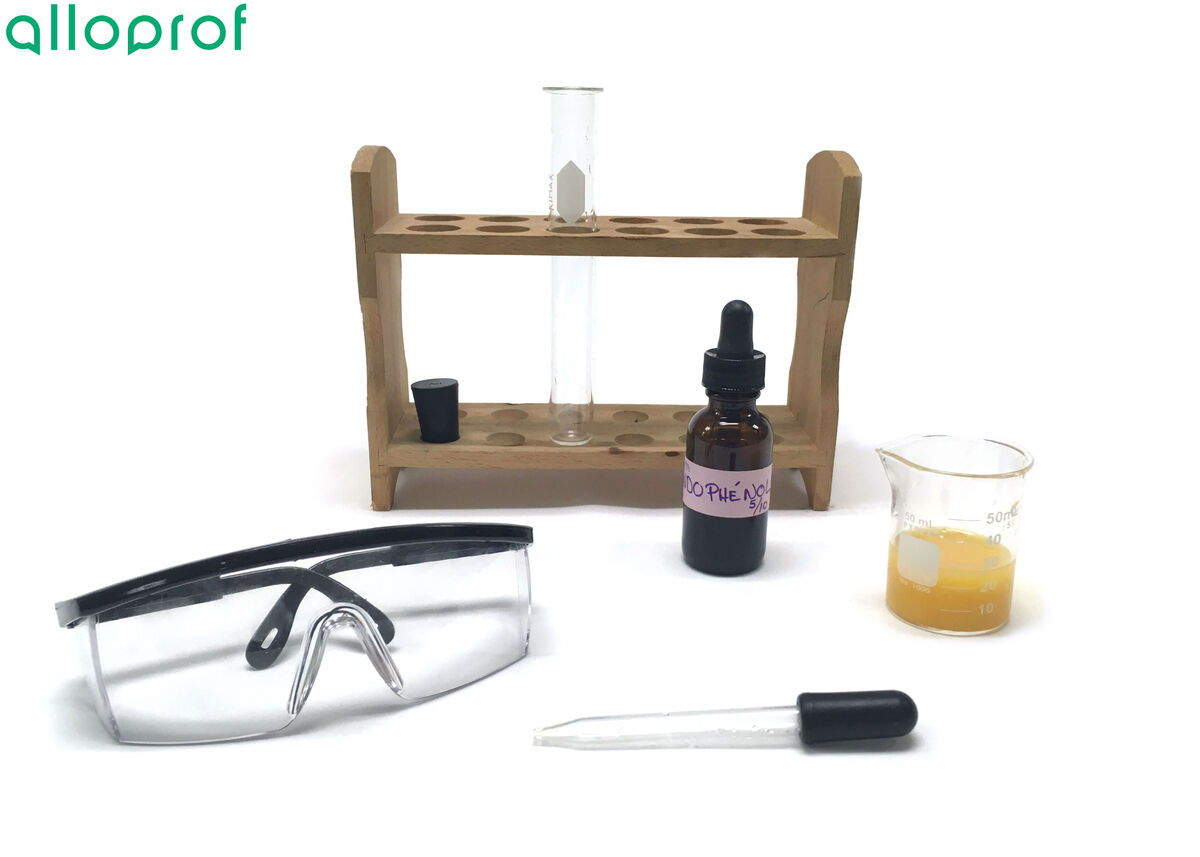
Indophenol
-
Apron or lab coat
-
Safety glasses or goggles
-
Measure 20 drops of the food and put them into a test tube.

-
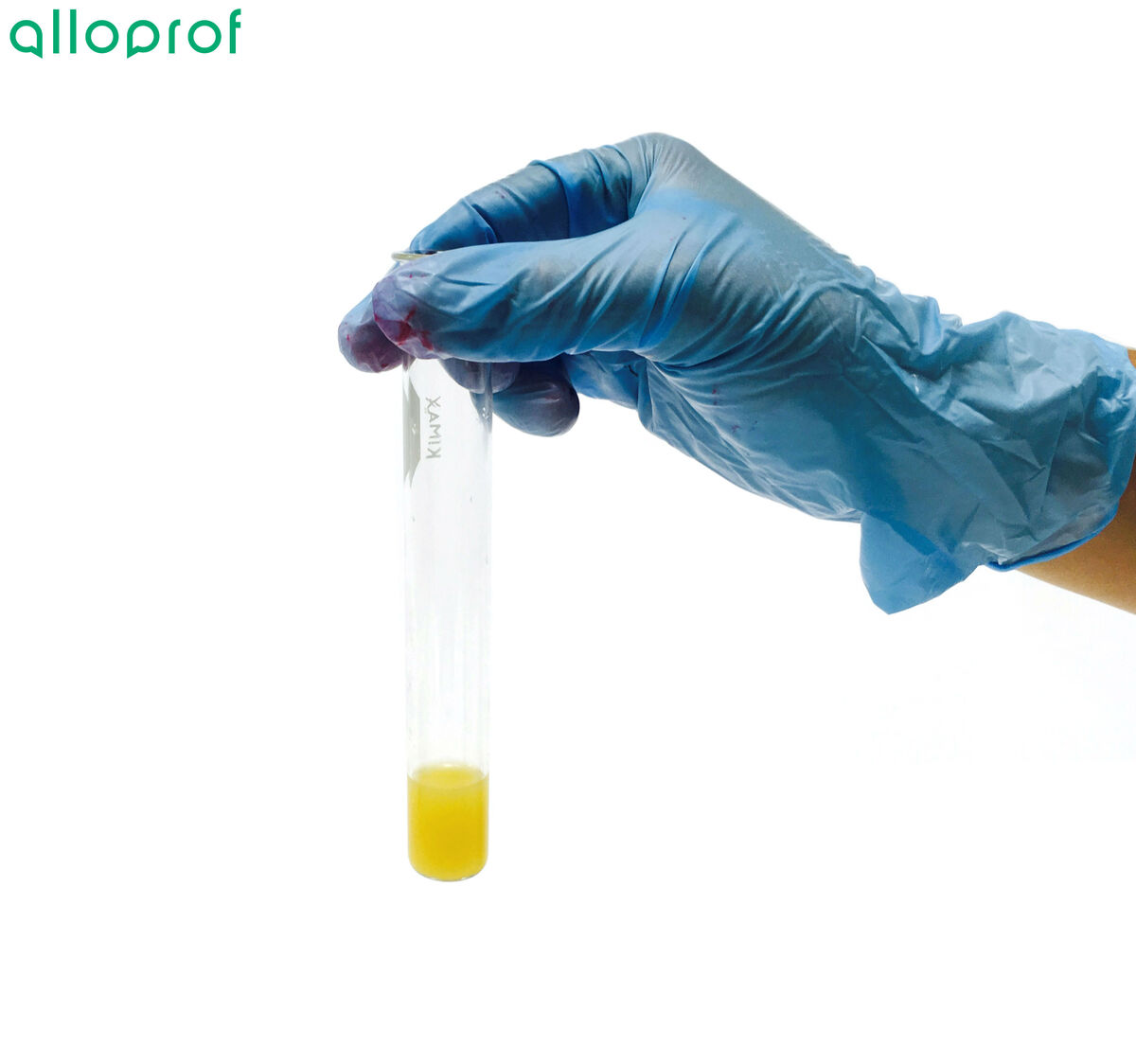
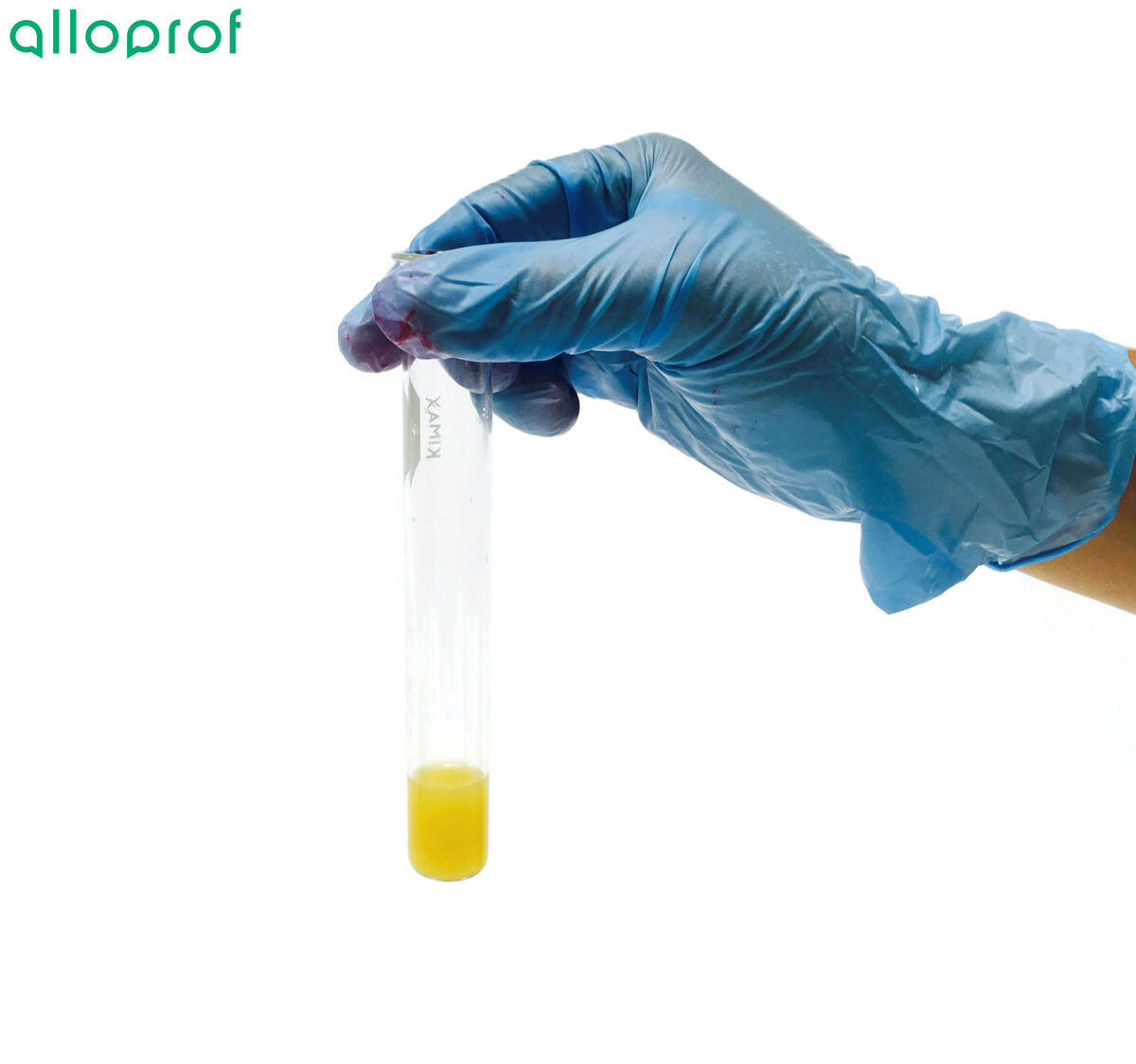
Place two drops of indophenol into the test tube.

-
Observe the colour obtained.
-
Clean and store the equipment.
There are two possible results for the indophenol test.
-
If the solution is a blue colour, the food does not contain vitamin C.

-
If the solution loses its blue colour and becomes colourless or yellowish, the food has vitamin C.
-
Test tube
-
Test tube rack
-
Dropper
-
Food in liquid form
-
Silver nitrate
-
Apron or lab coat
-
Safety glasses or goggles

-
Measure 20 drops of the food and put them into a test tube.

-
Place four drops of silver nitrate into the test tube.

Silver nitrate is a substance that can burn the skin. It is essential to be very careful when doing this test.
-
Observe the contents of the test tube.
-
Clean and store the equipment.
Analyze the results by observing whether or not there is the presence of a precipitate in the solution.
-
If there is no precipitate in the solution, this means that the food does not have mineral salts containing chloride.

-
If a precipitation reaction occurs, then there are inorganic salts containing chloride in the solution.
-
Test tube
-
Test tube rack
-
Dropper
-
Food in liquid form
-
Ammonium oxalate
-
Apron or lab coat
-
Safety glasses or goggles

-
Measure 10 drops of the food and put them into a test tube.

-
Place 10 drops of ammonium oxalate into the test tube.

-
Observe the contents of the test tube.

-
Clean and store the equipment.
Determine the results of the test by observing whether or not there is the presence of a precipitate in the solution.
-
If there is no precipitate in the solution, the food does not contain calcium.

-
If a precipitation reaction occurs, the food contains calcium.
