The buffering capacity of a soil is the ability of certain soils to resist changes in pH.
The pH of a soil determines its degree of acidity or basicity. A pH value below 7 characterizes an acidic soil, while a pH value above 7 refers to a basic soil (also called alkaline). The pH varies according to the content of carbon dioxide, mineral salts, and organic matter in the soil. It plays an essential role in the microbiological activity of the soil, in supplying plants with water and in the absorption of nutrients by the roots.
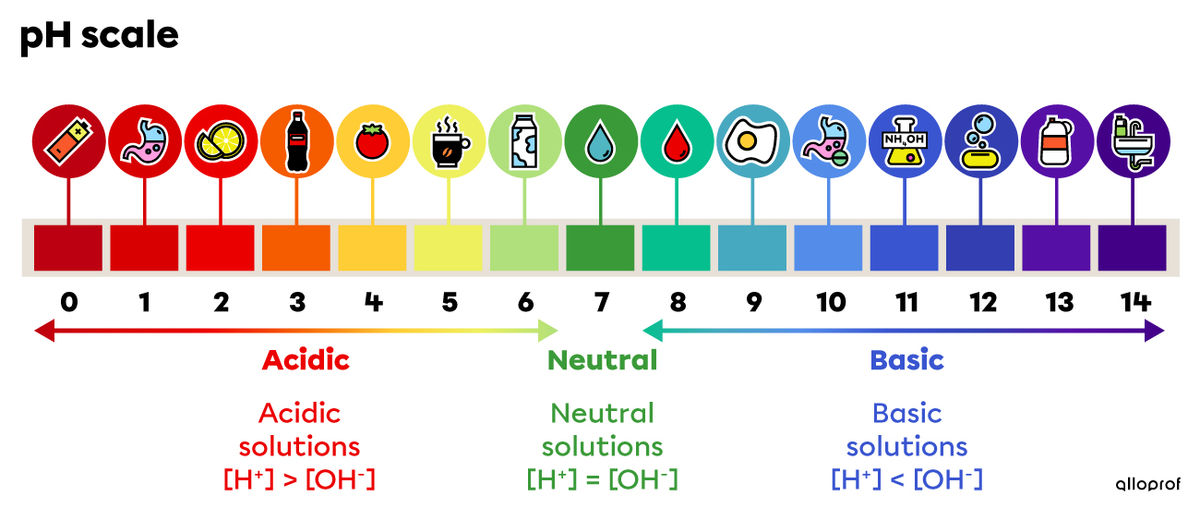
In order for plants to grow, a soil should not be too acidic or too basic — this would not allow plants to absorb the nutrients in the soil. Optimal plant growth is possible in a soil with a pH between 6 and 7, that is, in almost neutral, slightly acidic soil. It should be noted that some plant species have specific growth requirements. This is the case with conifers which grow better in more acidic soils.
Under certain climatic conditions, soils sometimes tend to acidify. Depending on their composition and mineral nature, more basic soils can react to changes in pH by neutralizing acidity. This chemical reaction is called the buffering effect. For example, a calcareous soil contains a large proportion of calcium carbonate, a basic mineral. It can therefore chemically neutralize acids. The variations in pH in this type of soil will therefore be less significant. In general, sandy soils have a hard time neutralizing acidity, while fertile soils rich in humus and minerals have a good buffering capacity. They can, therefore, neutralize the acidity of rain, allowing these soils to retain sufficient richness to provide plants with essential nutrients.
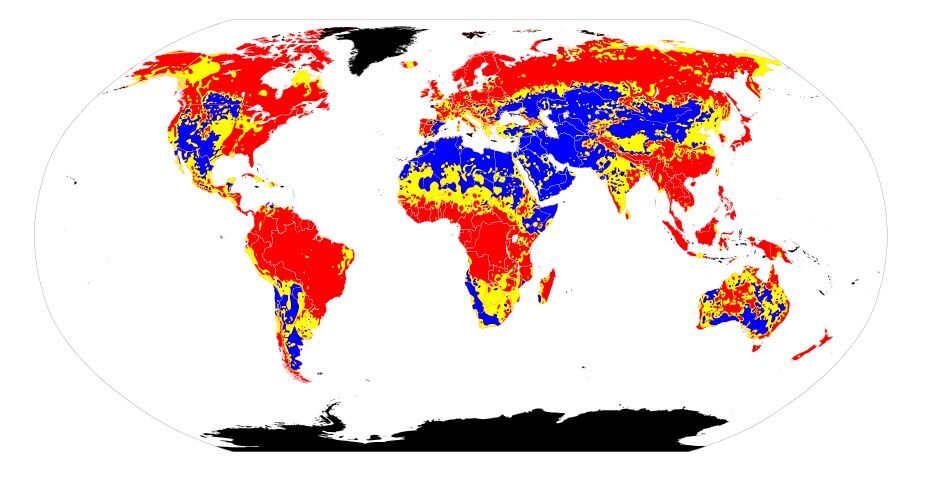
The following map shows the acidity of soils on the Earth's surface. Areas in red represent areas where the soil is acidic. Areas in yellow have neutral soils, while blue areas represent basic soils. If the area is black, no data is available to determine the soil acidity.