Chemical equilibrium is a state in which the rate of the forward reaction is equal to that of the reverse reaction.
In everyday life, the term equilibrium is used to describe the state of something that is immobile, something that does not change. For example, some rock structures defy the laws of gravity by forming a stack in equilibrium. This type of equilibrium is called static equilibrium because it characterises the state of things that are immobile. However, objects in equilibrium are not always motionless. For example, a tightrope walker maintains his balance while moving on a wire. This is dynamic equilibrium.
Examples of static equilibrium (left) and dynamic equilibrium (right)
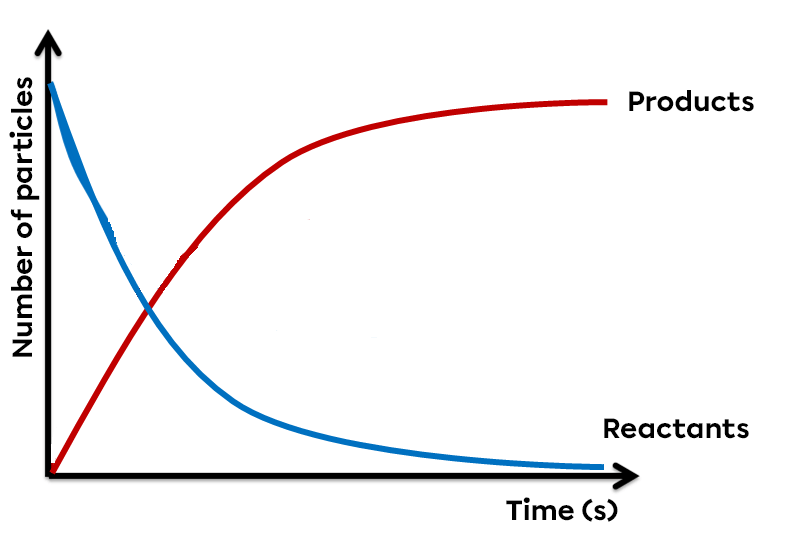
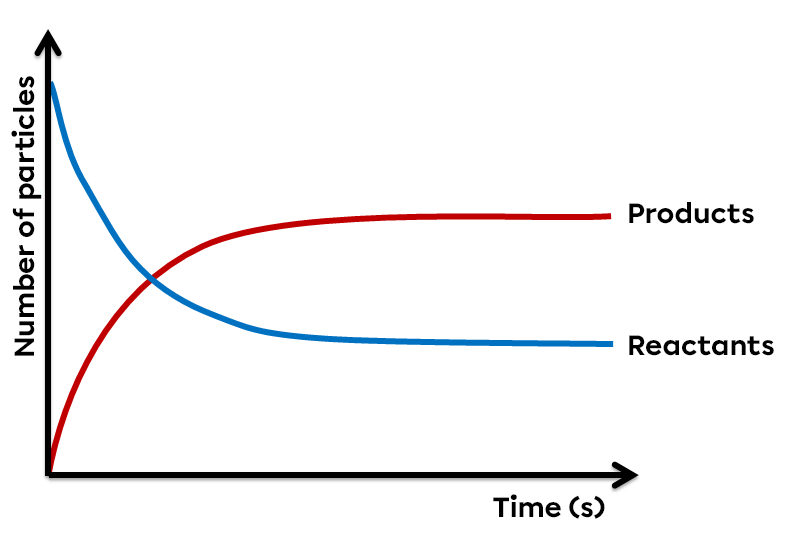
In chemistry, some reactions, rather than proceeding completely, reach a state of equilibrium. Even if, at first glance, no transformation appears to be taking place, there is in fact a perpetual exchange between the reactants and products involved. The reaction then reaches a dynamic equilibrium.
An irreversible, or complete, reaction is one that can only occur in one direction, from the reactants to the products. It occurs when at least one of the reactants has been completely transformed into the products.
A reversible, or incomplete, reaction is one that can occur in either direction. At the same place and at the same time, the reactants are transformed into products while the products are transformed into reactants.
Some reactions can only take place in the forward direction. For example, when wood is burnt, it reacts with oxygen in the air to produce energy and is transformed into ash and smoke. Ash and smoke cannot react to form wood again: this is an irreversible reaction. The decomposition of food is another example of a reaction that cannot be reversed. A reaction is therefore irreversible when it is complete and one or more of the reactants have been completely transformed.
However, some reactions can proceed in both the forward and reverse directions. Since there is a constant to-and-fro between the molecules of the reactants and those of the products, no substance has disappeared. It is therefore an incomplete reaction in which the forward reaction and the reverse reaction proceed simultaneously at the same speed. In a reversible reaction at equilibrium, the quantity of products and reactants remains constant and no change is apparent.
In an irreversible reaction (left), at least one of the reactants is completely transformed: the reaction is complete. In a reversible reaction (right), the quantity of products and reactants reaches a state of equilibrium.
Irreversible and reversible reactions are written differently. In the case of an irreversible chemical reaction, a one-way arrow running from left to right indicates that the reactants become entirely products and that the reaction is complete. For a reversible reaction, a double arrow is used to indicate that the reaction can proceed in either direction.
Irreversible chemical reaction: |CH_{4(g)} + 2\; O_{2(g)} \rightarrow CO_{2(g)} + 2\; H_{2}O_{(g)}|
Reversible chemical reaction: |H_{2(g)} + I_{2(g)} \rightleftharpoons 2\; HI_{(g)}|
The state of dynamic equilibrium may be physical or chemical. In chemistry, there are generally two types of physical equilibrium, phase equilibrium and solubility equilibrium, as well as chemical equilibrium.
Phase equilibrium occurs when a single substance is in several phases at the same time and place.
Phase equilibrium involves physical transformations that change phases. For example, despite winter temperatures, the water in a lake does not freeze completely. Only a layer of ice forms on the surface, while the bottom of the water remains in liquid form. The freezing process is therefore not complete, as the initial liquid is still present. The lake is therefore in a state of equilibrium, with water in two phases simultaneously.
The evaporation of water in a closed bottle at a constant temperature is another example of phase equilibrium. The water in the bottle evaporates while the water vapour condenses simultaneously.
Examples of phase equilibrium: lake water that does not freeze at depth (left) and the simultaneous liquid and gaseous phases in a bottle of water (right).
Solubility equilibrium is observed when a solution is saturated and a solute deposit appears.
All solutes have a maximum solubility. Beyond this saturation point, the solute no longer dissolves and instead settles to the bottom of the solution. It's easy to think that nothing is happening because the solute is no longer dissolving. On the contrary, the solution has reached a state of equilibrium. The excess solute is continually dissolving in the solvent, while the dissolved part reverts to a solid state. These two opposing processes take place at the same speed and simultaneously, resulting in a state of equilibrium.
Example of saturated chemical solutions
Chemical equilibrium occurs when two opposite chemical reactions take place at the same time and at the same speed.
Chemical equilibrium is more complex than the other two types of dynamic equilibrium, since it involves several substances and their transformations. At least two different substances must be present at the same time: a reactant and a product. Sometimes chemical equilibrium cannot be observed with the naked eye. In this case, it is necessary to check whether the conditions necessary for obtaining a state of equilibrium are present, or to rely on the description of the chemical reaction (i.e. to find the double arrow).
Although the transformation is not visible to the naked eye, nitrogen peroxide gas (colorless) is constantly being transformed into nitrogen dioxide (brownish), and vice versa. The color difference observed here is attributable to the pressure difference present in the two bottles. The chemical equation for this reaction is written as follows: |2\; NO_{2(g)} \rightleftharpoons N_{2}O_{4(g)}|