The state or the phase of matter refers to the organization of its particles.
The main states of matter are solid state, liquid state and gaseous state. The state of a substance varies according to several factors, including temperature. The following image shows different states of water.
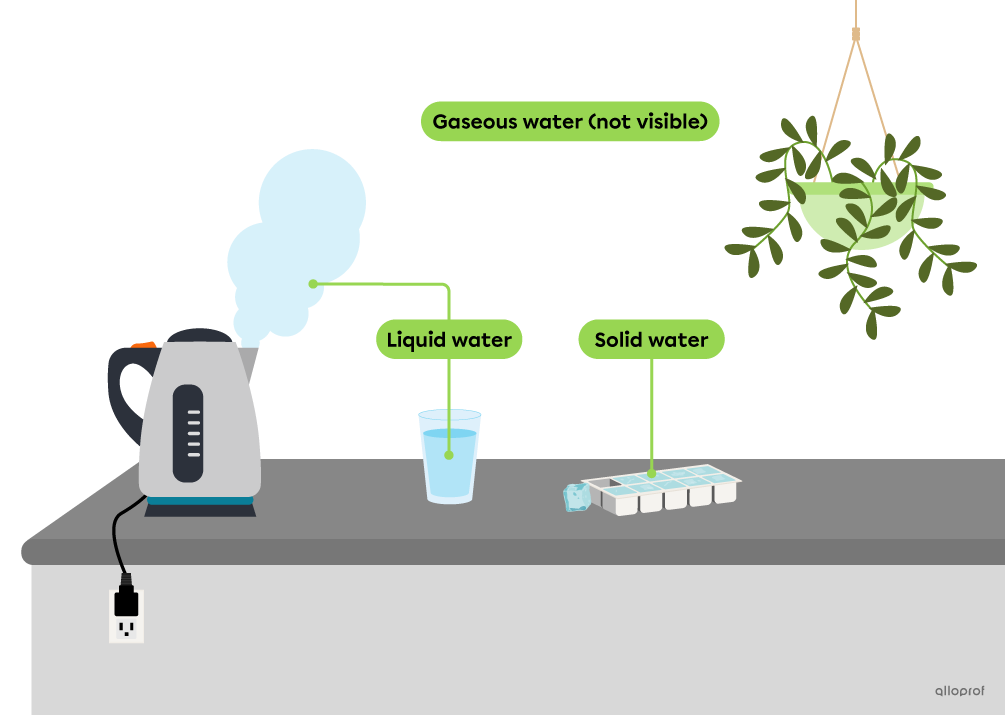
Water exists in gaseous state in the air, but it is not visible to the naked eye.
The cloud of hot water coming out of the kettle is in liquid state. It is composed of very fine droplets of liquid water suspended in the air. The water in the glass is also in a liquid state.
The ice cubes are in solid state.
It is possible to distinguish the solid, liquid and gaseous states of a substance with the naked eye. It is also possible to distinguish them at the particle scale. The particles of solids, liquids and gases behave differently. The distance between the particles and their organization are properties that vary according to the state of a substance.
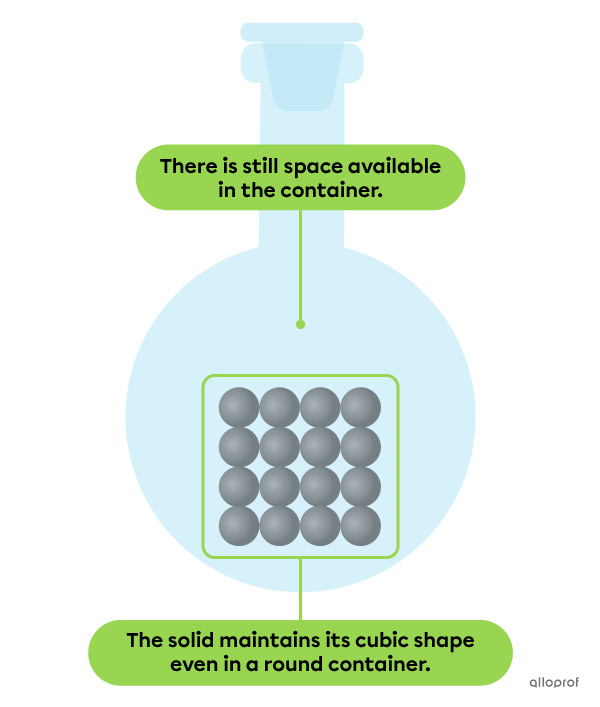
The particles of a substance in solid state are very strongly bound together. As a result, they are very close to each other and also highly organized.
A solid has a definite shape. This means that it retains its shape no matter what container it’s in.
Also, a solid has a definite volume. This means that it does not necessarily occupy all the space available in its container.
Chocolate chunks, volcanic rocks and aluminum cans are substances in solid state.
Sand is a granular material, meaning that it is made up of many small solid grains that are not bound together. On a large scale, sand seems to behave like a liquid, since it takes the shape of its container. This can be observed in an hourglass, for example. However, each individual grain has the properties of a solid. These grains have a definite shape and volume.

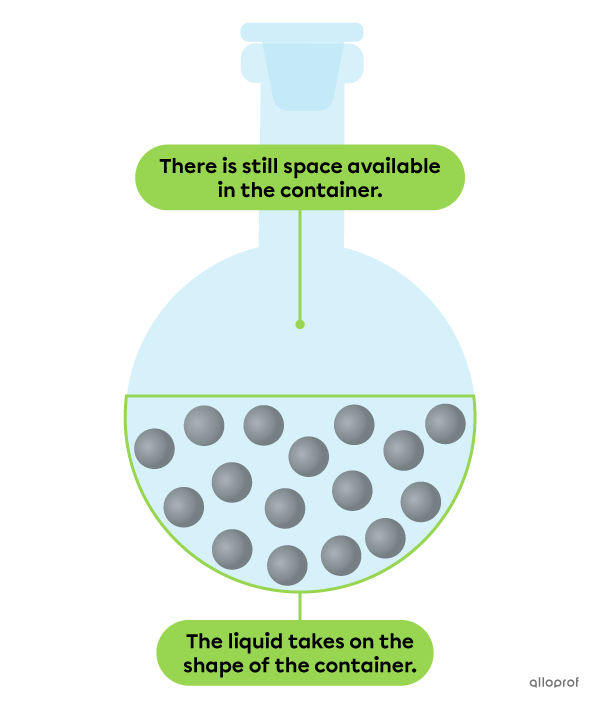
The particles of a substance in a liquid state are less strongly bound together. As a result, they stay close together, but are not very organized.
A liquid does not have a definite shape. This means that it tends to spread or take on the shape of its container.
However, a liquid has a definite volume. This means that it does not necessarily occupy all the space available in its container. It always occupies the same space, no matter what container it is poured into.
Honey, water in clouds and rainwater as well as motor oil are substances in liquid state.
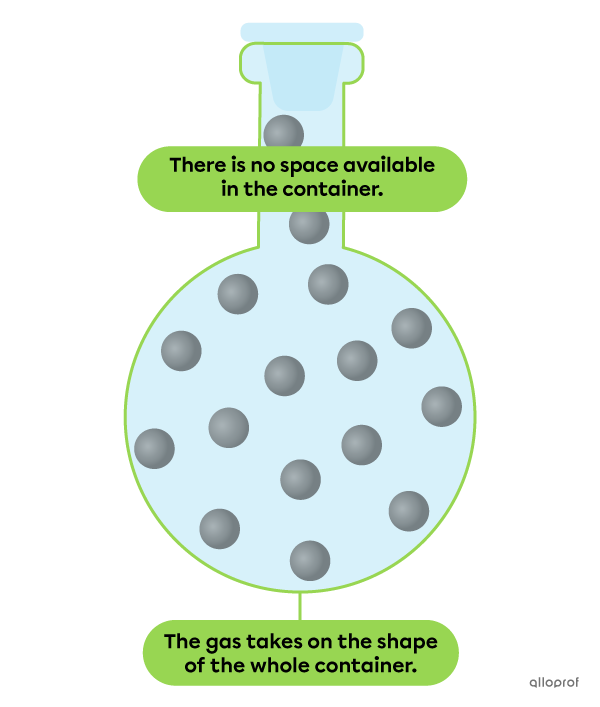
The particles of a substance in gaseous state are very loosely bound together. This means that they are far apart and not organized at all.
A gas does not have a definite shape. This means that it tends to take the shape of the container.
Also, a gas does not have a definite volume. This means that it occupies all the space available in its container.
The bubbles of carbon dioxide in sparkling water, the air we breathe and the gas emissions produced by a car are examples of substances in gaseous state.
Pour valider ta compréhension à propos des états de la matière de façon interactive, consulte la MiniRécup suivante :









