To predict the effects of concentration variation on the equilibrium state (whether it be the concentration of reactants or the concentration of products), we simply need to follow Le Chatelier's principle. A concentration variation of a single substance can disturb an equilibrium state. Based on Le Chatelier's principle, the consequences of concentration variation can be summarised as follows:
When the concentration of a reactant or product is changed, the reaction at equilibrium tends to oppose the change.
-
An increase in the concentration of a reactant tends to favour the forward reaction.
-
An increase in the concentration of a product tends to favour the reverse reaction.
-
A decrease in the concentration of a reactant tends to favour the reverse reaction.
-
A decrease in the concentration of a product tends to favour the forward reaction.
To understand the effects of a concentration variation on the equilibrium state, let's consider the following example:
|N_{2(g)} + 3\; H_{2(g)} \rightleftharpoons 2\; NH_{3(g)}.|
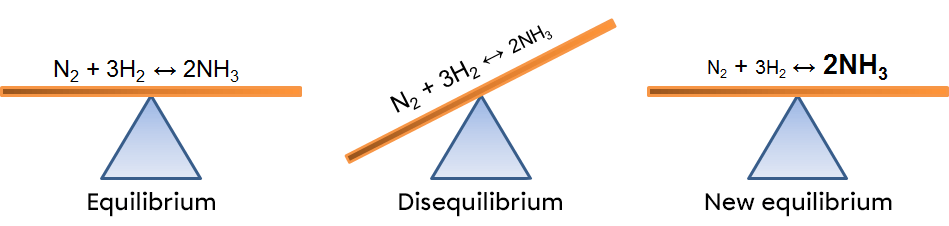
According to Le Chatelier's principle, a system in equilibrium reacts to partially oppose the changes imposed on it. As a result, when there is an increase in the concentration of a reactant, the system will react in the opposite way, favouring the direction of the reaction using this excess of reactant. In this case, the forward reaction.
In this example, an increase of nitrogen creates an imbalance which favours the forward reaction. To return to a new equilibrium state, the system opposes the change by using the excess reactant. It thus favours the forward reaction, causing a decrease in reactants and an increase in products. The effects of such a variation can be illustrated in two ways:
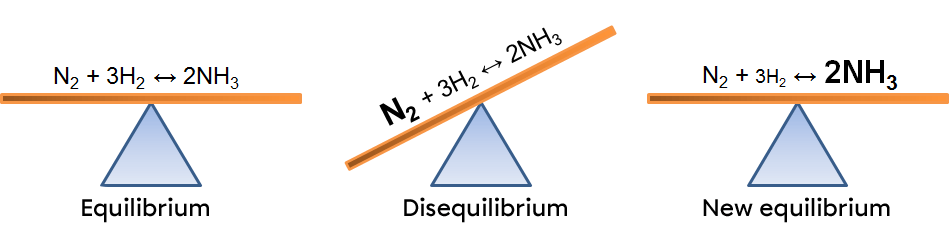
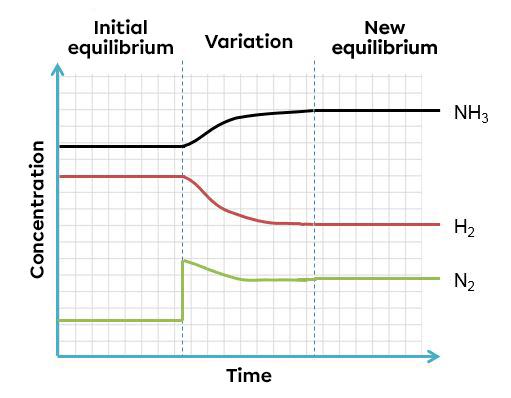
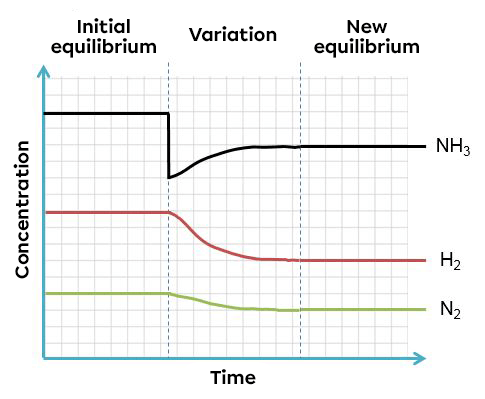
According to Le Chatelier's principle, a system in equilibrium reacts to partly oppose the change imposed on it. As a result, when there is an incease in the product concentration, the system will react in the opposite way, favouring the direction of the reaction using this excess of product. In this case, the reverse reaction.
In this example, an increase of ammonia creates an imbalance which favours the reverse reaction. To return to a new equilibrium state, the system opposes the change by using this excess product. It thus favours the reverse reaction, causing an increase in reactants and a decrease in products. The effects of such a variation can be illustrated in two ways:
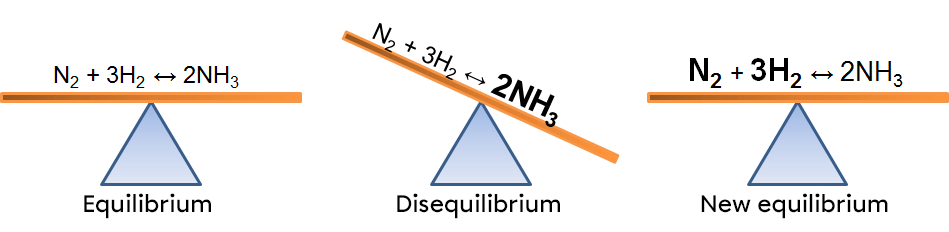
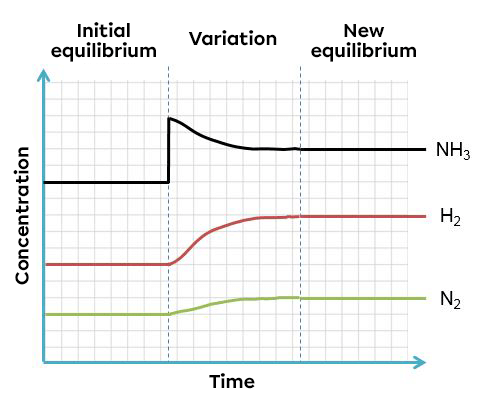
According to Le Chatelier's principle, a system in equilibrium reacts to partly oppose the changes imposed on it. As a result, when there is a decrease in the concentration of a reactant, the system will react in the opposite way, favouring the direction of the reaction compensating for the lack of reactant. In this case, the opposite reaction.
In this example, a decrease of nitrogen creates an imbalance which favours the reverse reaction. To return to a new equilibrium state, the system opposes the change by compensating for the lack of reactant. It thus favours the reverse reaction, causing an increase in reactants and a decrease in products. The effects of such a variation can be illustrated in two ways:

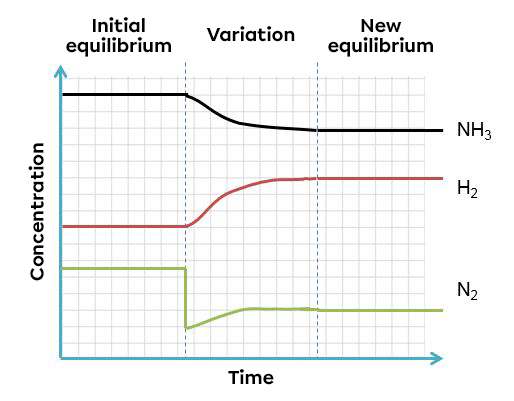
According to Le Chatelier's principle, a system in equilibrium reacts to partly oppose the changes imposed on it. As a result, when there is a decrease in the concentration of a product, the system will react in the opposite way, favouring the direction of the reaction that compensates for the lack of product. In this case, the forward reaction.
In this example, a decrease of ammonia creates an imbalance which favours the forward reaction. To return to a new equilibrium state, the system opposes the change by compensating for the lack of product. It thus favours the forward reaction, causing a decrease in reactants and an increase in products. The effects of such a variation can be illustrated in two ways:
|
Imposed change |
Schematics |
Favoured reaction |
|
Increase in reactants |
Forward reaction |
|
|
Decrease in reactants |
Reverse reaction |
|
|
Increase in products |
Reverse reaction |
|
|
Decrease in products |
Forward reaction |