Dalton's atomic model illustrates atoms as balls of different colours and sizes depending on the nature of the element.
This model is called Dalton's atomic theory. It is based on 4 important points.
| Important point of Dalton's theory | Example |
|
1. Matter is made up of small, invisible, indivisible particles called atoms. |
An oxygen atom cannot be seen or divided.
|
|
2. Atoms of a given element are identical. They have the same properties, and have the same mass. |
Oxygen atoms are all alike. They are the same size, and have the same mass and the same properties.
|
|
3. Atoms of different elements have different properties and masses. |
A carbon atom is different from an oxygen atom.
|
|
4. Atoms can combine to form a new substance. The produced molecule has different properties from the atoms that make it up. |
When we combine one carbon atom with two oxygen atoms, we get carbon dioxide (or carbonic acid gas).
|
To put Dalton's atomic model in its historical context and to learn more about the different atomic models, refer to the concept sheet on the history of the atomic model.
In Dalton's atomic model, atoms of different elements are illustrated with balls of different colours and sizes. This allows them to be easily seen in a drawing. The following colours are usually used to represent hydrogen, carbon, nitrogen and oxygen.
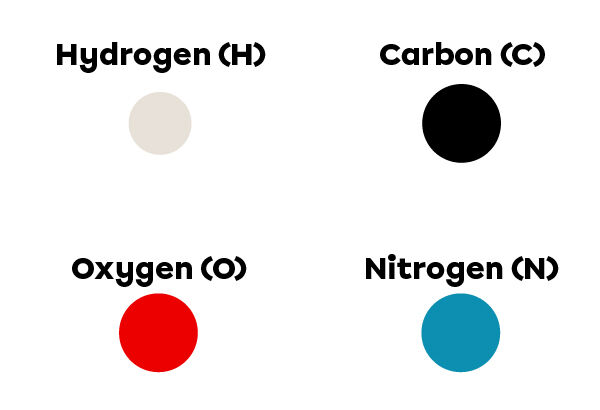
To represent a molecule, we must first understand its chemical formula. Upper case letters, sometimes followed by lower case letters, correspond to the elements present in the molecule. The numbers appearing as subscripts, meanwhile, correspond to the number of each element’s atoms present in the molecule. If there is no subscript, it means that the molecule contains only one atom of this element. Simply draw each element in a different way and make sure that the right amount is represented.
To draw a |\bf{C\color{Red}{O_2}}| molecule, it is first necessary to identify the different elements that compose it. The symbol |\bf{C}| corresponds to carbon and the symbol |\bf{\color{Red}{O}}|, to oxygen. According to the legend, carbon is illustrated with a black ball and oxygen with a red ball.
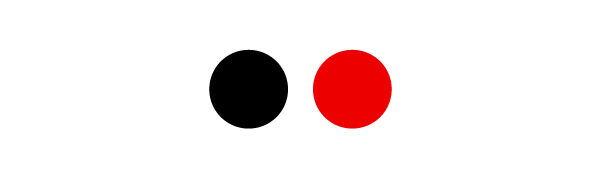
In |\bf{C\color{Red}{O_2}}|, there is no subscript number after the |\bf{C}|, therefore the molecule contains a single carbon atom. There is a subscript of 2 for the |\bf{\color{Red}{O}}|. The molecule then contains 2 oxygen atoms. So that the representation is correct, we draw a black ball illustrating the carbon atom and we connect two red balls representing the oxygen atoms.
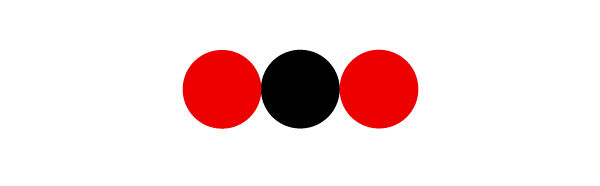
To find the chemical formula of a molecule, we must first identify the elements that constitute it using the legend provided. It is then necessary to determine the quantity of each element and to indicate it as a subscript in the chemical formula. If there is only one atom of a certain element, no subscript is added to this element.
Let’s find the chemical formula of this molecule:

To identify the elements present in the molecule, we refer to the following legend:
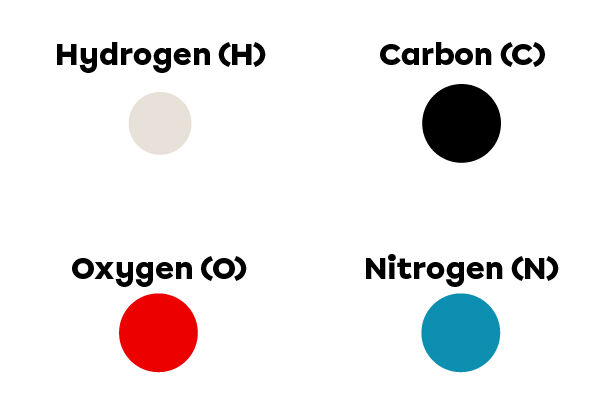
In the molecule, there is 1 black ball and 1 red ball. This means that it contains 1 atom of carbon and 1 atom of oxygen.
The molecule is therefore illustrated as follows: |\text{CO}|.
Bensaada, A., Bolduc, A., Claude, V., Meziane, M., Rhéaume, C. et Tardif, K. (2012). Kaléidoscope ST-STE - 2e cycle (2e année) (2e éd.). Chenelière Éducation.
Couture, I. et Peyronnet, O. (2009). Synergie, 2e cycle (2e année). [Manuel de l'élève]. Chenelière Éducation.
Khan Academy. (2020). Le modèle atomique de Dalton. https://fr.khanacademy.org/science/chemistry/electronic-structure-of-atoms/history-of-atomic-structure/a/daltons-atomic-theory-version-2



