Choose your level.
The atom is a particle of matter invisible to the naked eye. It is the basic unit of the molecule.
Since the atom is invisible to the naked eye, it took a long time to prove its existence and determine its properties.
The oldest recorded reference to the atom is attributed to the Indian philosopher Kanada around 600 BC. He hypothesized the existence of indivisible particles called anu. Around 400 BC, the Greek philosopher Democritus also spoke of indivisible particles which he called atomos.
In the 18th century, the English chemist John Dalton developed a model of the atom based on experimental data. It is known as Dalton's atomic model. Although this model contains some errors, it helped in understanding many things about the properties of the atom and about chemical reactions.
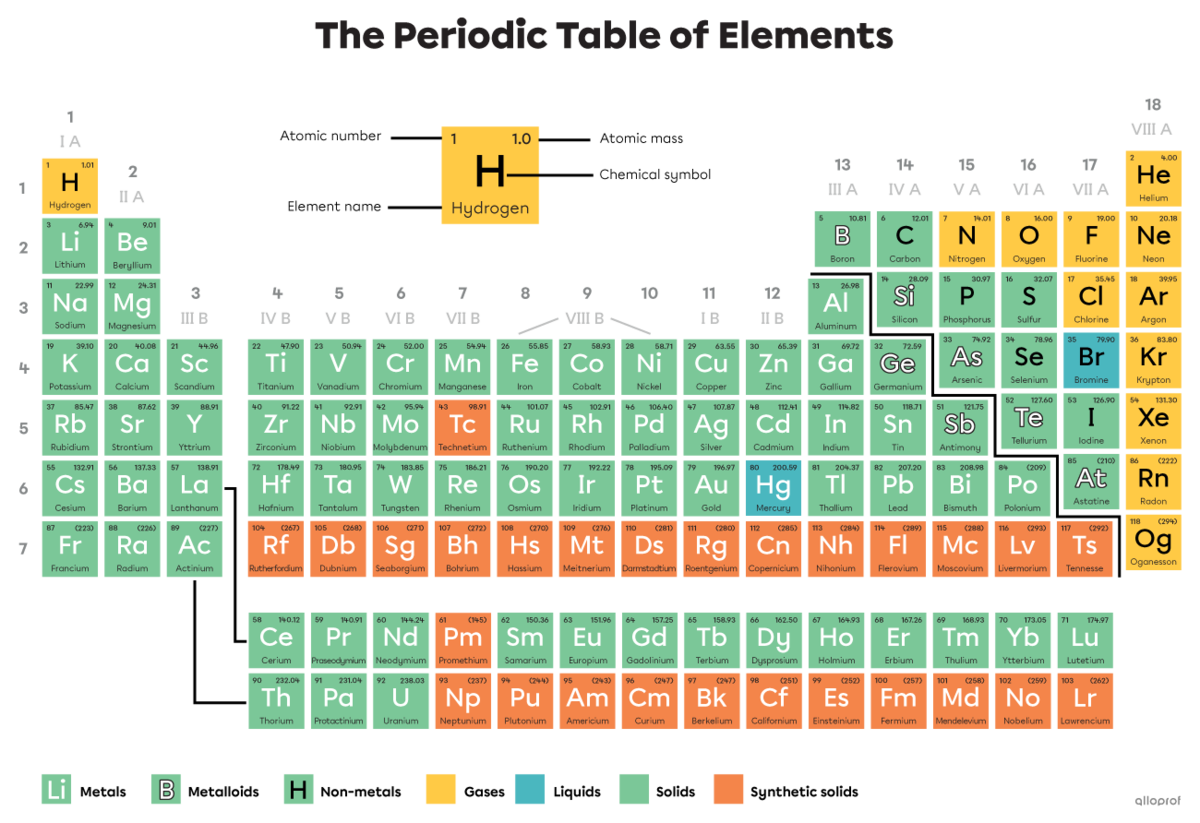
Today, 118 types of atoms are known. They are categorized in a table called the periodic table of elements.
Contrary to what John Dalton thought in the early 19th century, the atom is divisible. It consists of subatomic particles: protons, electrons and neutrons. The number of each subatomic particle varies according to the type of atom.
As the structure of the atom was gradually discovered, several atomic models were developed in the 20th century. Here are some examples.
Other models developed later showed that protons and neutrons are also divisible, and include even smaller particles.
Pour valider ta compréhension à propos de l’organisation de la matière de façon interactive, consulte la MiniRécup suivante.

The atom is a particle of matter invisible to the naked eye. It is the basic unit of the molecule, and of compounds in general.
Throughout history, many atomic models have been developed to explain the properties of atoms. Based on these models, it is known that atoms can combine to form different types of substances such as polyatomic elements, molecules or compounds. It was also established that atoms are divisible and that they include subatomic particles.
Today, 118 types of atoms are known. They are represented in an organized table called the periodic table of elements.
Subatomic particles are particles smaller than the atom.
Contrary to what John Dalton thought in the early 19th century, the atom is divisible.
The number of each subatomic particle varies according to the type of atom.
Electrons are indivisible. They are said to be elementary particles. However, protons and neutrons are divisible. They are composed of quarks. There are several types of quarks: up quarks, down quarks, strange quarks, etc. They are differentiated by their charge.
A proton contains 2 up quarks (|\dfrac{2}{3}+| charge) and 1 down quark (|\dfrac{1}{3}-| charge). The sum of the charges of the 3 quarks equals a value of |1+|, which corresponds to the charge of a proton.
A neutron also contains 3 quarks: 2 down quarks and 1 up quark. The sum of the charges of the 3 quarks equals a value of |0,| which corresponds to the charge of a neutron.
Electrons are negatively charged particles found around the nucleus of the atom.
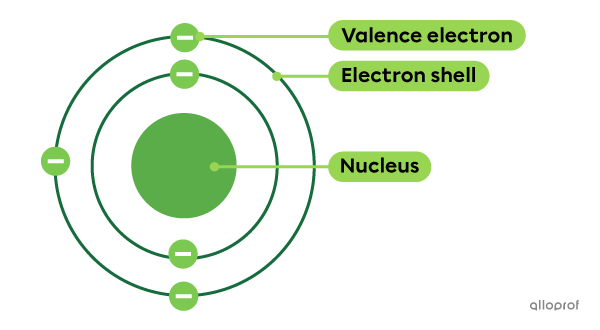
In the early 20th century, John Thomson demonstrated the existence of electrons and identified several of their properties. Later, Niels Bohr's experiments led to the development of a model according to which electrons are found on electron shells.
The electrons on the electron shell furthest from the nucleus are called valence electrons. They allow atoms to combine by forming chemical bonds.
Several properties of electrons are listed in the following table.
| Symbol | Charge | Mass[1] |
|---|---|---|
| |\text{e}^-| | |1-| | |9.109\times10^{-31}\ \text{kg}| |
Since an atom is neutral, it contains the same number of protons and electrons.
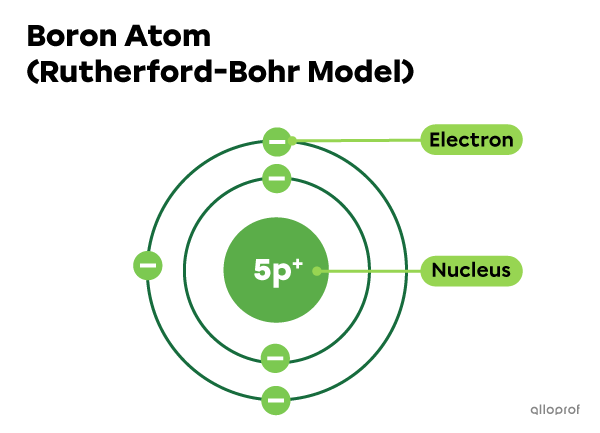
The boron atom |(\text{B})| has 5 protons in its nucleus. Since it is neutral, it has to have 5 electrons, as well.
At the beginning of the 20th century, scientists were convinced that electrons were nothing but particles. However, the work of Louis de Broglie, Clinton Davisson, Lester Germer and many others demonstrated that electrons are not only capable of behaving like particles, but also like waves! This phenomenon is referred to as the wave-particle duality.
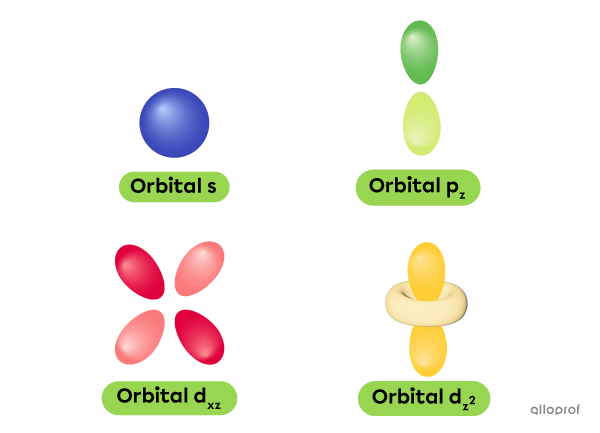
This discovery led to the quantum mechanical model of the atom. In this model, electrons are not found in circular orbits, like in the Rutherford-Bohr model. Instead, the electrons are found in probability zones called orbitals, which have different shapes and sizes, depending on the type of atom.
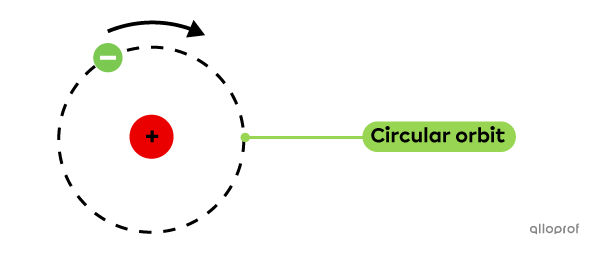
Protons are positively charged particles found in the nucleus of the atom.
At the beginning of the 20th century, John Thomson hypothesized the existence of protons. However, it was Ernest Rutherford who proved their existence and identified several of their properties.
Since protons are found in the nucleus of the atom, they are considered to be nucleons.
Several properties of protons are listed in the following table.
| Symbol | Charge | Mass[2] |
|---|---|---|
| |\text{p}^+| | |1+| | |1.673\times10^{-27}\ \text{kg}| |
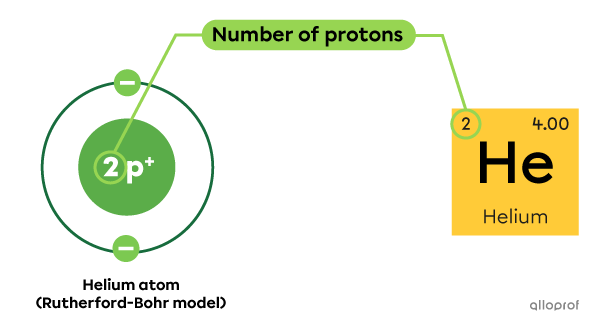
In the periodic table of the elements, the atomic number |(\text{Z})| indicated in the square of a given atom corresponds to its number of protons. The number of protons in the nucleus of an atom defines its identity.
Every helium atom |(\text{He})| has 2 protons in the nucleus.
As indicated in the periodic table, the atomic number |(\text{Z})| of helium is 2.
Which atom has 4 protons in the nucleus?
The atom with 4 protons in its nucleus is the atom whose atomic number in the periodic table is |4.| Therefore, it is the beryllium atom.

Along with neutrons, protons contribute significantly to the atomic mass of elements.
Neutrons are neutral particles found in the nucleus of the atom.
Numerous experiments on radioactivity eventually led to the discovery of neutrons. It was only in 1932 that James Chadwick revised the atomic model to include neutrons in the nucleus of the atom. Neutrons bind to protons to stabilize the nucleus by reducing the effect of the repulsive force between protons.
Since neutrons can be found in the nucleus of the atom, they are considered to be nucleons.
Of all the elements in the periodic table, only the nucleus of the hydrogen-1 atom |(_{1}^{1}\text{H})| does not contain any neutrons.
Several properties of neutrons are listed in the following table.
| Symbol | Charge | Mass[3] |
|---|---|---|
| |\text{n}^0| | |0| | |1.675\times10^{-27}\ \text{kg}| |
Unlike the number of protons |(\text{Z}),| the number of neutrons |(\text{N})| in the nucleus of an atom is not indicated in the periodic table. However, it can be calculated from the mass number |(\text{A}).|
Along with protons, neutrons contribute significantly to the atomic mass of the elements.
Ce bloc n'est nécessaire qu'en anglais.
-
The National Institute of Standards and Technology. (2018). Fundamental Physical Constants - Electron mass. https://www.physics.nist.gov/cgi-bin/cuu/Value?me|search_for=electron+m…
-
The National Institute of Standards and Technology. (2018). Fundamental Physical Constants - Proton mass. https://www.physics.nist.gov/cgi-bin/cuu/Value?mp|search_for=proton+mass
-
The National Institute of Standards and Technology. (2018). Fundamental Physical Constants - Neutron mass. https://www.physics.nist.gov/cgi-bin/cuu/Value?mn|search_for=neutron+ma…