Energy is not lost, it is transformed: this is the law of conservation of energy. It can change form indefinitely, because it undergoes transformations with the transfer of energy. During these numerous exchanges of energy, some reactions release energy and others absorb it.
Exothermic reactions are reactions which release energy, thus increasing the energy level of their environment. This can be noticeable by an increase in temperature or by the release of light.
When a chemical reaction releases heat in a medium, the temperature of this medium increases. The final temperature is therefore higher than the initial temperature.
In a chemical equation, a reaction is recognized as exothermic when the energy value is written on the product side of the reaction — to the right of the arrow.
|N_{2(g)} + 3 \space H_{2(g)} \rightarrow 2 \space NH_{3(g)} + 95.4 kJ|
This reaction can also be expressed by placing the energy variable outside the chemical equation. However, the change in energy, expressed as the change in enthalpy |(\triangle H)|, is conventionally preceded by a negative sign (-). The negative sign indicates that there is a loss of energy.
|N_{2(g)} + 3 \space H_{2(g)} \rightarrow 2 \space NH_{3(g)} \space \space \triangle H = - 95.4 kJ|
Here is a diagram representing the exothermic reaction of ammonia synthesis |(NH_{3})| and the evolution of energy during this reaction.
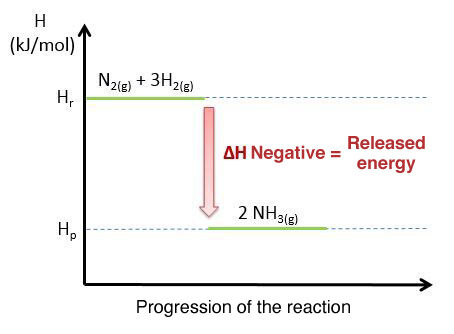
The above graph illustrates an exothermic reaction, as the enthalpy of the products is at a lower level than the enthalpy of the reactants.
There are several examples of exothermic reactions in chemistry. Most combustions, slow or fast, and neutralization reactions are exothermic reactions.
Endothermic reactions are reactions that lower the energy level of the medium by absorbing energy. This can be seen by a drop in the medium’s temperature.
When a chemical reaction absorbs heat in a medium, the temperature of that medium decreases. The temperature at the end of the reaction is therefore lower than at the start. The surrounding environment is therefore responsible for this energy transfer.
In a chemical equation, a reaction is recognized as endothermic when the energy value is written on the reactant side of the equation — to the left of the arrow.
|2 \space NH_{3(g)} + 95.4 kJ \rightarrow N_{2(g)} + 3 \space H_{2(g)}|
This reaction can also be expressed by placing the energy variable outside the chemical equation. The change in energy, or change in enthalpy |(\triangle H)|, is conventionally preceded by a positive sign (+), a sign which indicates that there is an energy gain.
|2 \space NH_{3(g)} \rightarrow N_{2(g)} + 3 \space H_{2(g)} \space \space \triangle H = + 95.4 kJ|
Here is a diagram showing the endothermic reaction of the decomposition of ammonia and the evolution of energy during this reaction.
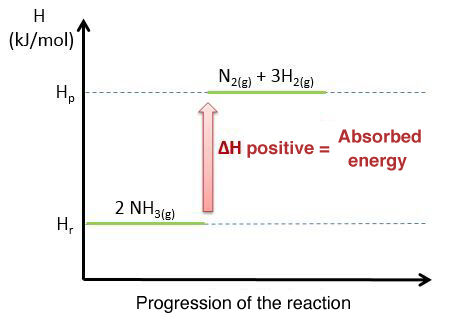
The above graph illustrates an endothermic reaction, as the enthalpy of the reactants is at a lower level than the enthalpy of the products.
Several examples of endothermic reactions can be found, including most chemical decompositions, whether by the input of heat, light, or electricity (electrolysis).