This section explains the procedure to follow to determine the nature of certain gases.
Gases are sometimes difficult to identify. However, with the help of simple tests, it is easy to recognize oxygen gas |\left( O_2 \right)|, hydrogen gas |\left( H_2 \right)|, and carbon dioxide gas |\left( CO_2 \right)|. In fact, each of these gases behaves in a different way when brought close to a flame, glowing splint, or limewater.
There are three tests that can help identify the nature of the gases.
The flame test is used to identify the presence of hydrogen gas |\left( H_2 \right)| in a container.
- Test tube with stopper containing the unknown gas
- Test tube rack
- Wood splint
- Matches
- Lab coat
- Safety glasses

Since gases are often lighter than air, it is recommended to leave the test tube upside down in the test tube rack to prevent gas from escaping.

1. Light a wood splint to obtain a bright flame.

It is important to work safely when handling matches.
2. Open the test tube containing the gas and quickly insert the flaming wood splint.

As mentioned earlier, it is preferable to hold the test tube upside down (stopper down) to ensure that all gas remains inside the test tube.
If possible, it is recommended to repeat the experiment a second time to validate the experimental results obtained the first time.
If a small explosion is heard, it could be concluded that the unknown gas is hydrogen.
However, for any other reaction, the gas is not hydrogen. Further tests are then required to confirm the identity of the gas.
The flame may go out during this test. Although carbon dioxide |\left( CO_2 \right)| can extinguish the flame, it is recommended to carry out a limewater test in order to validate this observation.

The glowing splint test is used to identify the presence of oxygen gas |\left( O_2 \right)| in a container.
- Test tube with stopper containing the unknown gas
- Test tube rack
- Wood splint
- Matches
- Lab coat
- Safety glasses

Since gases are often lighter than air, it is recommended to leave the test tube upside down in the test tube rack to prevent gas from escaping.

1. Light a wood splint.
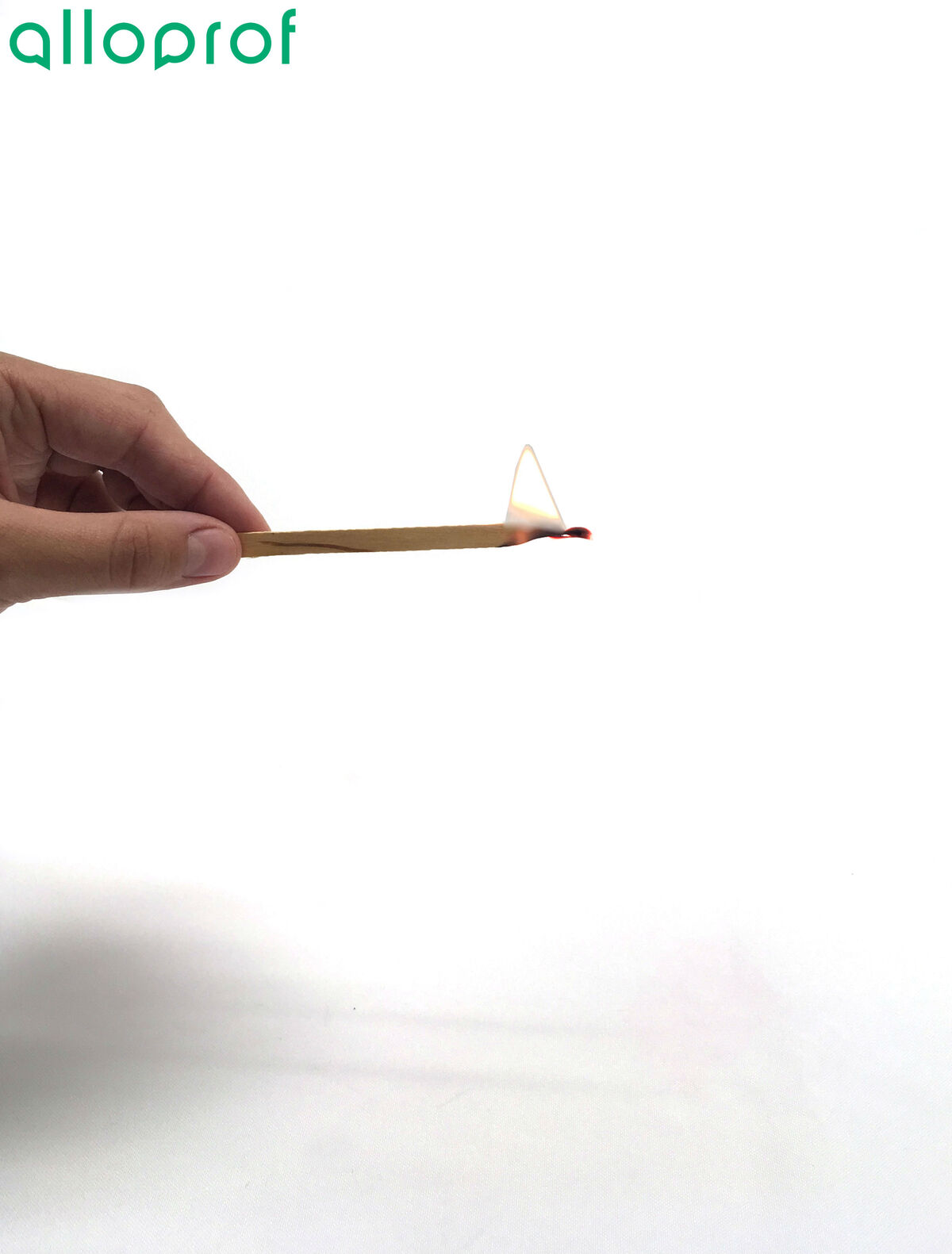
It is important to work safely when handling matches.
2. Extinguish the wood splint to obtain a glowing splint.
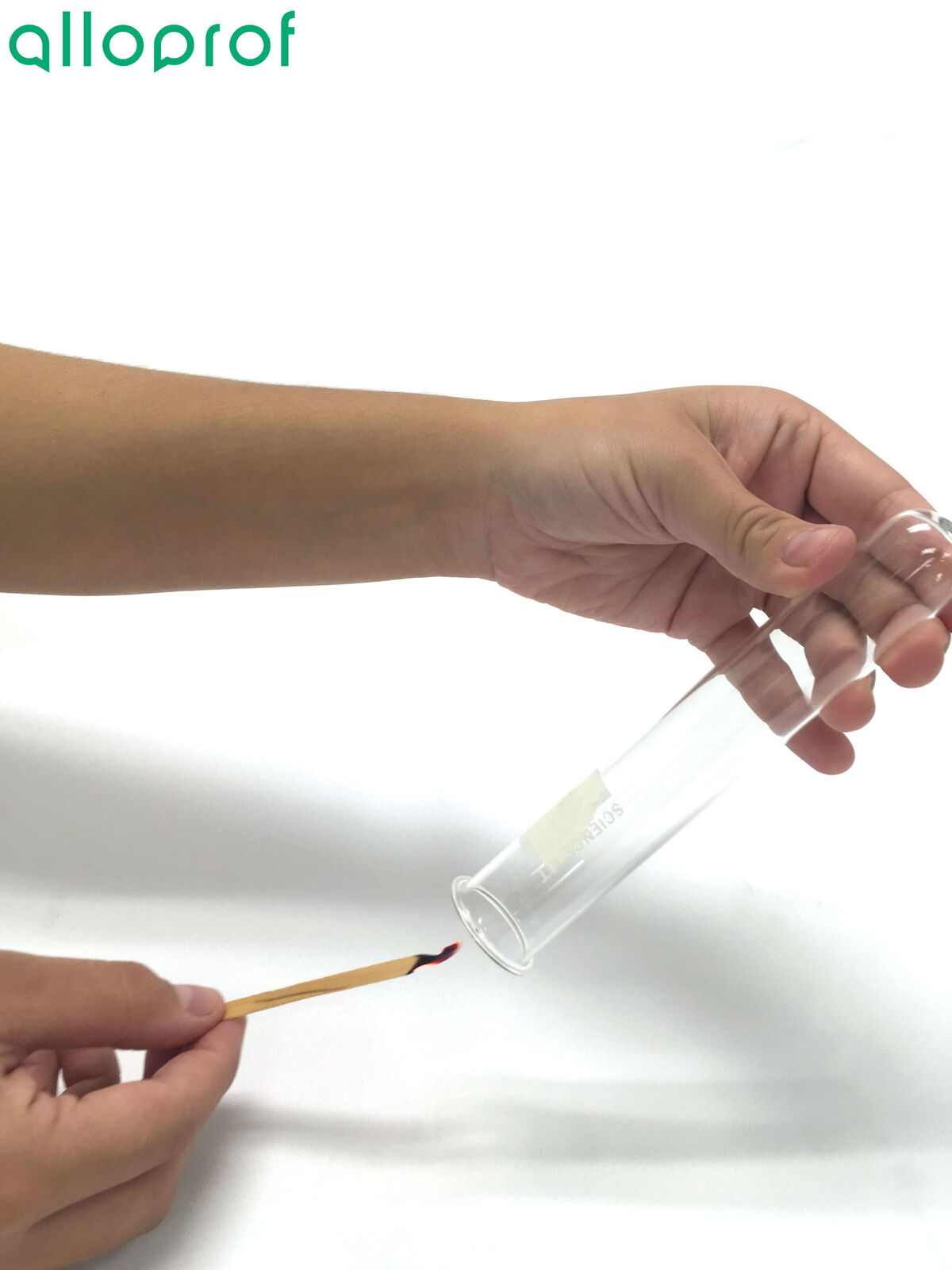
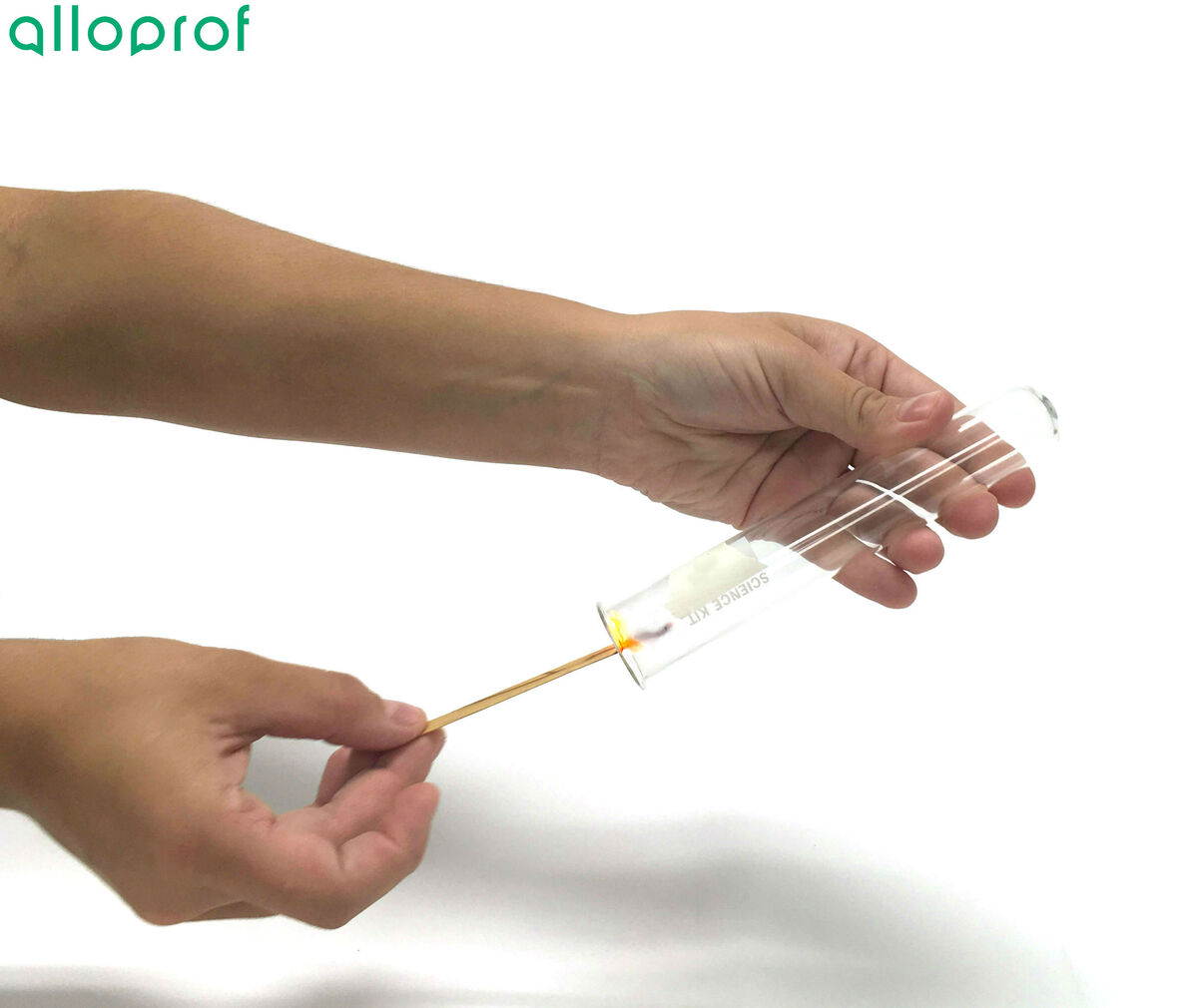
3. Open the test tube containing the gas and quickly insert the glowing splint.

As mentioned earlier, it is preferable to hold the test tube upside down (stopper down) to ensure that all gas remains inside the test tube.
If possible, it is recommended to repeat the experiment a second time to validate the experimental results obtained the first time.
If the glowing splint reignites, it can be concluded that the unknown gas is oxygen.

However, for any other reaction, the gas is not oxygen. Further tests are then required to confirm the identity of the gas.
The limewater test is used to identify the presence of carbon dioxide |\left( CO_2 \right)| in a container.
- Test tube with stopper containing the unknown gas
- Test tube rack
- Dropper
- Limewater |\left( CaCO_3 \right)|
- Lab coat
- Safety glasses

Since gases are often lighter than air, it is recommended to leave the test tube upside down in the test tube rack to prevent gas from escaping.

1. Insert approximately 2 to 3 mL of limewater into the test tube containing the gas to be identified.

2. Quickly close the test tube before shaking.

If the liquid turns white or milky, it could be concluded that the unknown gas is carbon dioxide.

However, if the liquid remains colourless, the gas is not carbon dioxide. Further tests are then required to confirm the identity of the gas.
