The chemical characteristic properties make it possible to identify a pure substance by means of a chemical reaction that will change the nature of the substance.
Chemical characteristic properties are often studied through the use of chemical indicators such as the indicators used to determine pH. This type of property is also observed in gas identification tests.
The colour of neutral litmus paper indicates the acidity of a substance.
If litmus paper turns red, the substance is acidic (it has a pH below 7).
If litmus paper turns blue, the substance is basic (it has a pH above 7).
If neither paper changes colour (red litmus paper stays red and blue litmus paper stays blue), the substance is neutral (it has a pH of 7).
Cobalt chloride paper indicates whether there is water in a solution.
If the cobalt chloride paper turns pink, the solution contains water.
This reaction indicates the presence of carbon dioxide (CO2).
If the limewater becomes cloudy and forms a precipitate on contact with a gas, it means that carbon dioxide is present.
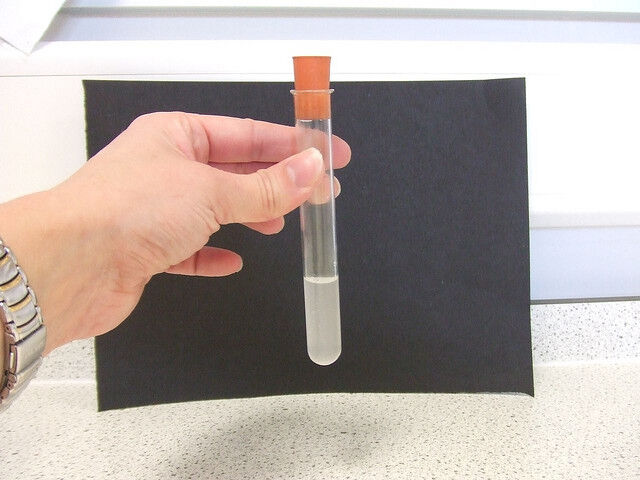
The glowing splint reaction makes it possible to see if a substance likely to create combustion, usually oxygen, is present.
If the glowing splint reignites the flame, there is a substance present that can generate combustion.
This reaction indicates the presence of an explosive gas, usually hydrogen.
If there is an explosion with a flaming splint, the substance contains an explosive gas.
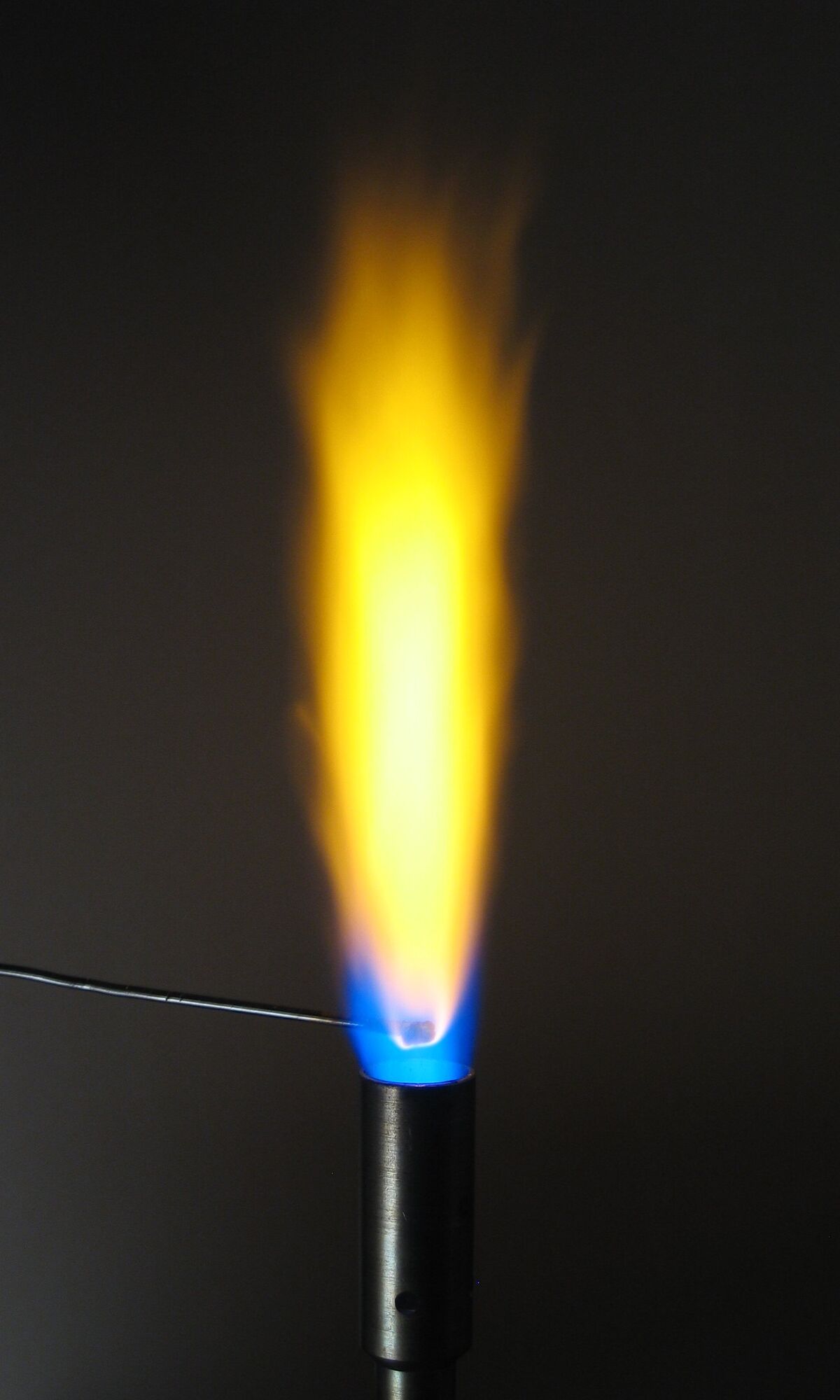
The colour of the flame indicates the presence of different substances.
If the flame turns yellow, the substance may contain sodium.
If the flame turns green, the substance may contain copper.

If the flame turns red, the substance may contain lithium.

If the flame turns pink, the substance may contain potassium.

If a substance turns brown when heated in the presence of Fehling's solution, there are carbohydrates in the solution.
When a substance turns purplish in the presence of sodium hydroxide |(NaOH)| and copper sulphate |(CuSO_{4})|, there are proteins in the solution.


