It was Antoine Laurent de Lavoisier who formulated the law conservation of matter:
During transformation, matter is neither created nor destroyed, but is transformed from its initial state to a final state.
“Nothing is lost, nothing is created, all is transformed.”
This law indicates that the number of atoms of each type is the same before and after a transformation. The same is true for mass. The mass of the reactants is the same as the mass of the products.
In a physical change, the mass of the reactants and the products remains the same, because they contain the same atoms, molecules and/or compounds at the beginning as at the end of the reaction.
This principle can be easily observed by weighing a closed beaker filled with ice and weighing it again after the ice melts. The total mass does not change.
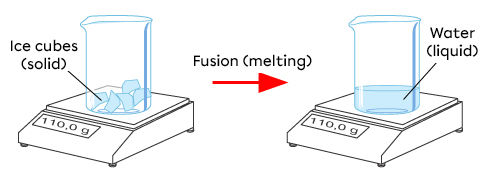
The same principle applies to chemical changes. The mass of the reactants and the products remains the same, because they contain the same atoms at the beginning and end of the reaction. The atoms rearrange to form new substances at the end of the reaction.
When heating copper powder (orange) in the presence of oxygen, the result is a more granular black powder. The oxygen |(\text{O}_2)| in the ambient air combines with the copper atoms |(\text{Cu})| to form copper oxide |(\text{CuO}).| The reaction is shown in the image below.
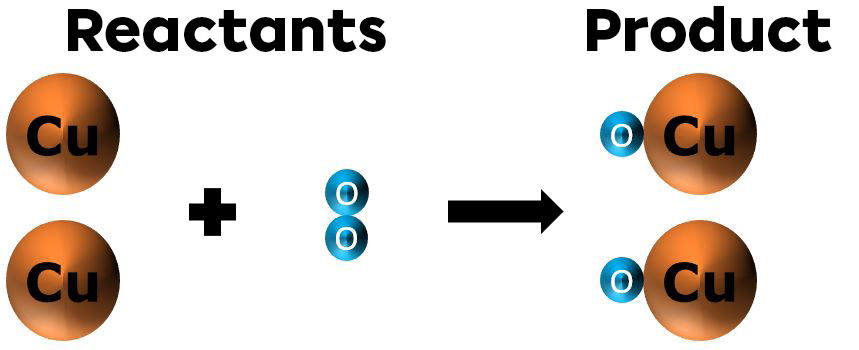
The matter is conserved (nothing is lost).
There are two copper atoms on the reactants side and two copper atoms on the products side.
There are also two oxygen atoms on the reactants side and two oxygen atoms on the products side.
Therefore, the mass is conserved. When the atoms are heated, new bonds form between the atoms. They create a new substance that does not have the same properties as the original substances.
The principle stated by Lavoisier indicates that the mass of reactants is equal to the mass of the products.
During a chemical reaction, 16 g of methane |(\text{CH}_4)| are burned with 64 g of oxygen gas |(\text{O}_2),| producing 36 g of water vapour |(\text{H}_2\text{O})| and some carbon dioxide |(\text{CO}_2).| What is the mass of carbon dioxide?
First, the chemical reaction must be written. Then, the mass of each reactant and product is noted under each substance.
|\text{CH}_4 + 2\ \text{O}_2 \rightarrow \text{CO}_2 + 2\ \text{H}_2\text{O}|
|16\ \text{g} + 64\ \text{g}\ \rightarrow\ {x} \ + 36\ \text{g}|
The total mass of the reactants is 80 g (16 g + 64 g). Following the principle of conservation of mass, the products must have the same mass as the reactants, or 80 g.
Since there are 36 g of water in the products, the mass of the |(\text{CO}_2)| must be: |80\ \text{g} - 36\ \text{g} = 44\ \text{g}.|
The mass of the |(\text{CO}_2)| formed in the reaction is 44 g.