This concept sheet explains the procedure for determining the measurement of a substance’s electrical conductivity.
Electrical conductivity is a property that tells us how easily electric currents can flow through a substance. To determine electrical conductivity, a device called an electrical conductivity detector (ECD) is used. The ECD is made up of two electrodes (usually copper). These are connected by an electrical circuit powered by a square battery (|9| V).


There are different models of ECD: some simply indicate the passage of electric current by turning on a light, while others have a scale indicating conductivity. The latter are more precise: a substance with a high electrical conductivity lights up to |10|, while a substance with a low electrical conductivity only lights up to |1|. It is, therefore, possible to know how well a substance allows electric current to flow.

Regardless of the ECD model used, the procedure for determining the conductivity of a substance is the same.
-
Substance to test (solid or liquid)
-
Electrical conductivity detector (ECD)
-
Safety glasses

1. Bring the two electrodes of the ECD into contact with the substance to be tested.


2. Observe the indicator light.
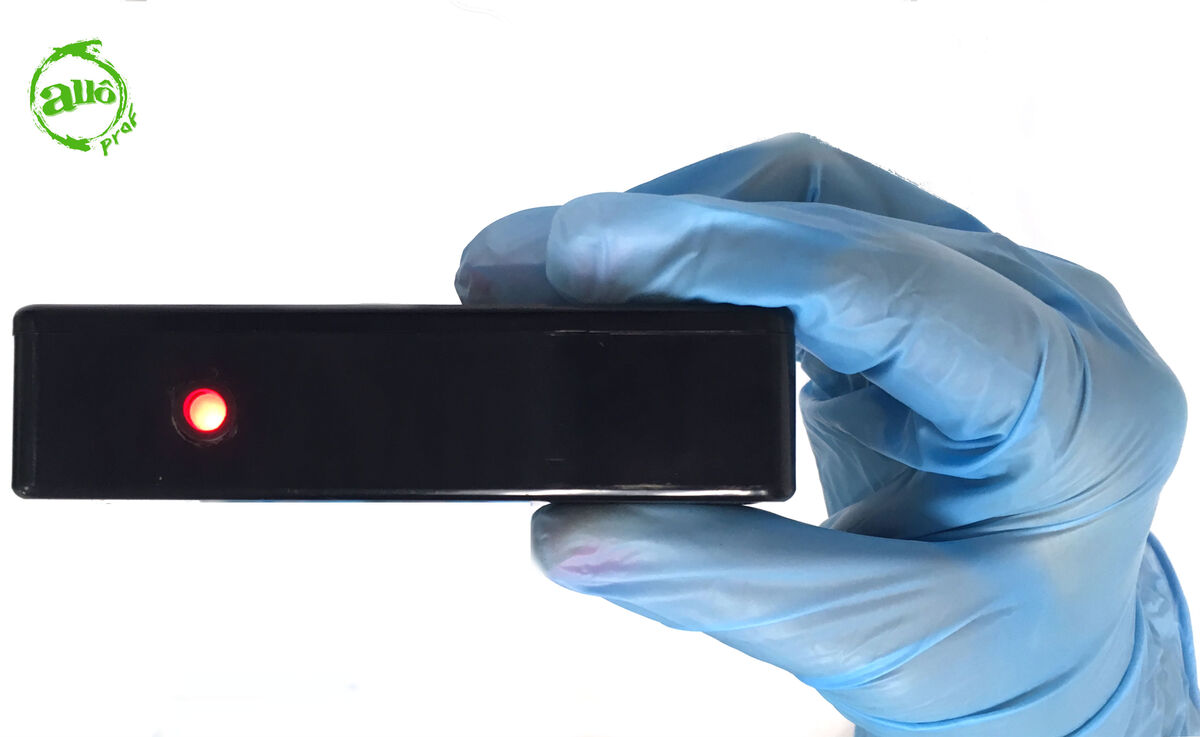

3. Put away the equipment.
When the ECD is used with liquid substances, it is important to clean the electrodes thoroughly to remove all traces of the liquid and to wipe them properly to avoid electrode degradation.
There are only two possible outcomes.
-
If the light turns on, the substance is an electrical conductor.
-
If the light does not turn on, the substance does not conduct electricity: it is therefore an insulator.

It is therefore possible to classify substances into different groups.
Among solids, there are two major groups of elements on the periodic table that conduct electricity: metals and metalloids. Substances that do not conduct electricity in the periodic table are non-metals. In addition, various compounds can be electrically conductive depending on the elements that make up these objects.
In liquids, acids, bases, and salts are the three main groups of substances that allow electric current to flow, because they are electrolytes. Substances that do not conduct electricity, such as alcohols or pure water, are non-electrolytes.
Tap water allows electric current to flow, because salts are dissolved in the water to facilitate electrical conductivity. However, if the salts are removed from the water (as in pure water or distilled water), then the liquid loses its capacity to provide electrical conductivity.