This concept sheet explains the procedures to follow in order to determine how the mass of a substance is measured.
When weighing a substance or object, it is its mass that is measured, not its weight. Therefore, a substance to be weighed is a substance whose mass is unknown and will be determined with a balance.
Mass is the amount of matter contained in an object or substance. When it is to be measured, it must be done with as much precision as possible. To achieve this, appropriate measurement techniques are used depending on the state of matter.
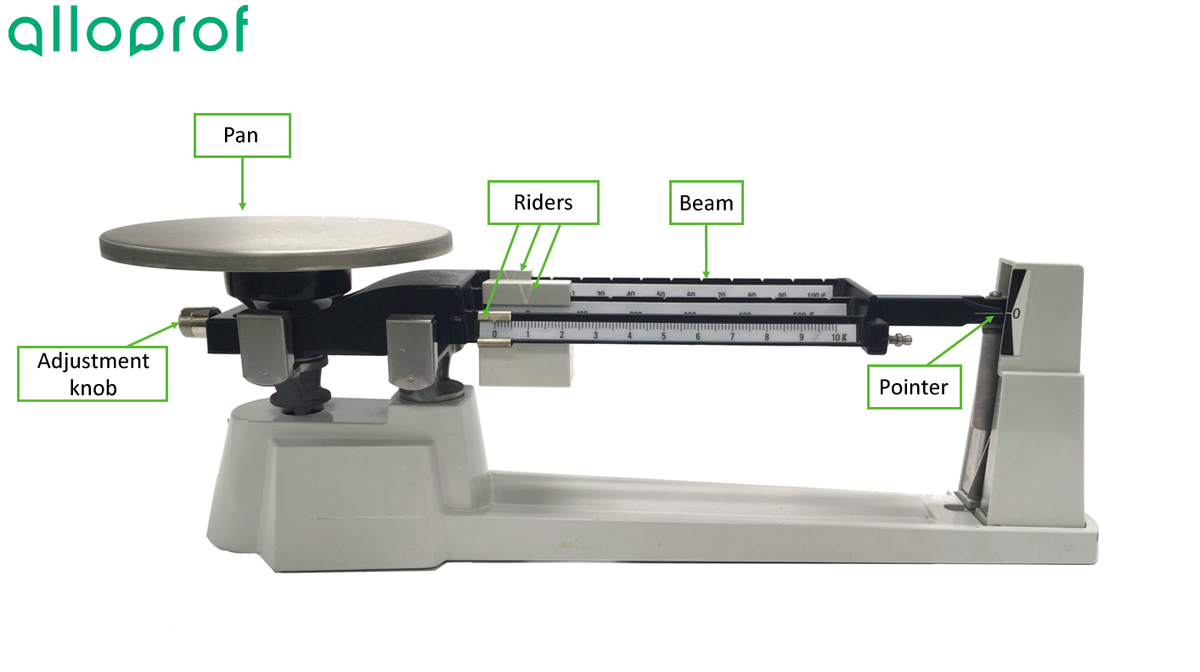
Here are the different parts of a triple beam balance:
In a quadruple beam balance, there are four beams and the pan hangs on a hook. When this type of balance is used, it is important to secure the pan on the hook correctly to prevent it from touching the base of the balance.

Moreover, these balances are industrially calibrated, which means that each pan is designed for one specific balance. It is therefore important to ensure that the pan number (located under the pan) corresponds to the balance number.


The preferred method for measuring mass is the same regardless of the state of the object.
1. Set the riders of the balance to zero. Make sure the pan is clean.

Make sure that the riders are correctly placed in the beam notches.
Also, the scale should not be placed on a table that is not level, and should not be placed in contact with other objects, such as the counter or glassware.
2. Check that the pointer is set to zero. If the pointer is not aligned with the zero, calibrate the balance with the adjustment knob.

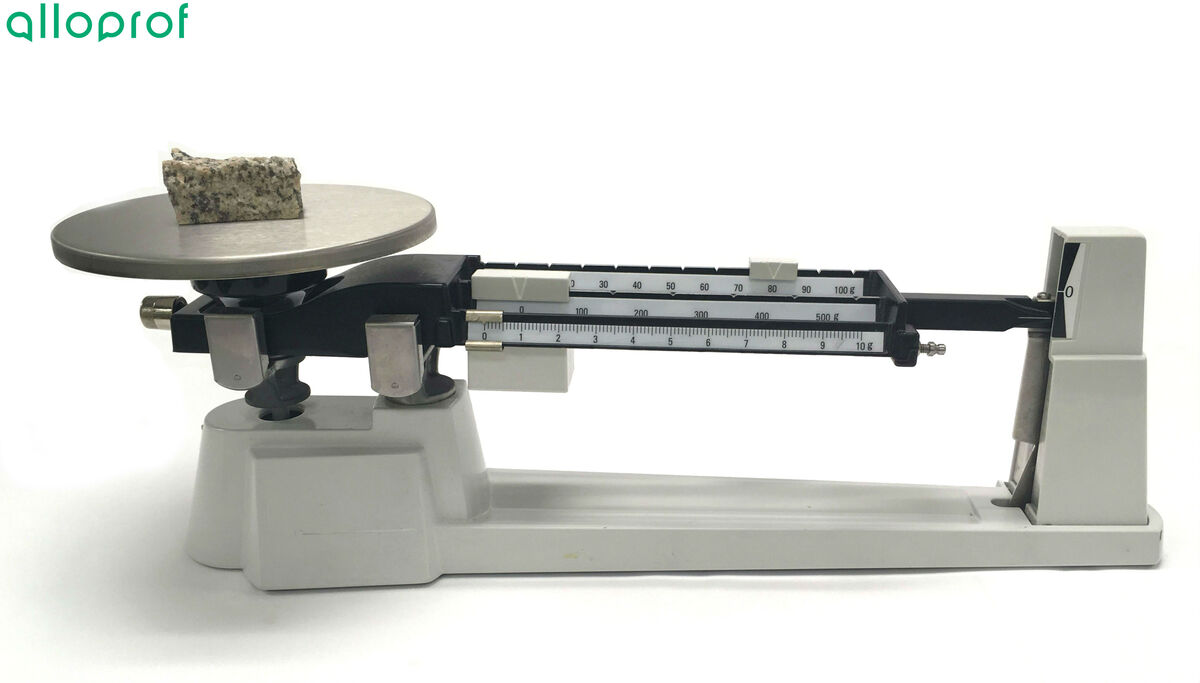
3. Place the object to be weighed on the pan of the balance.

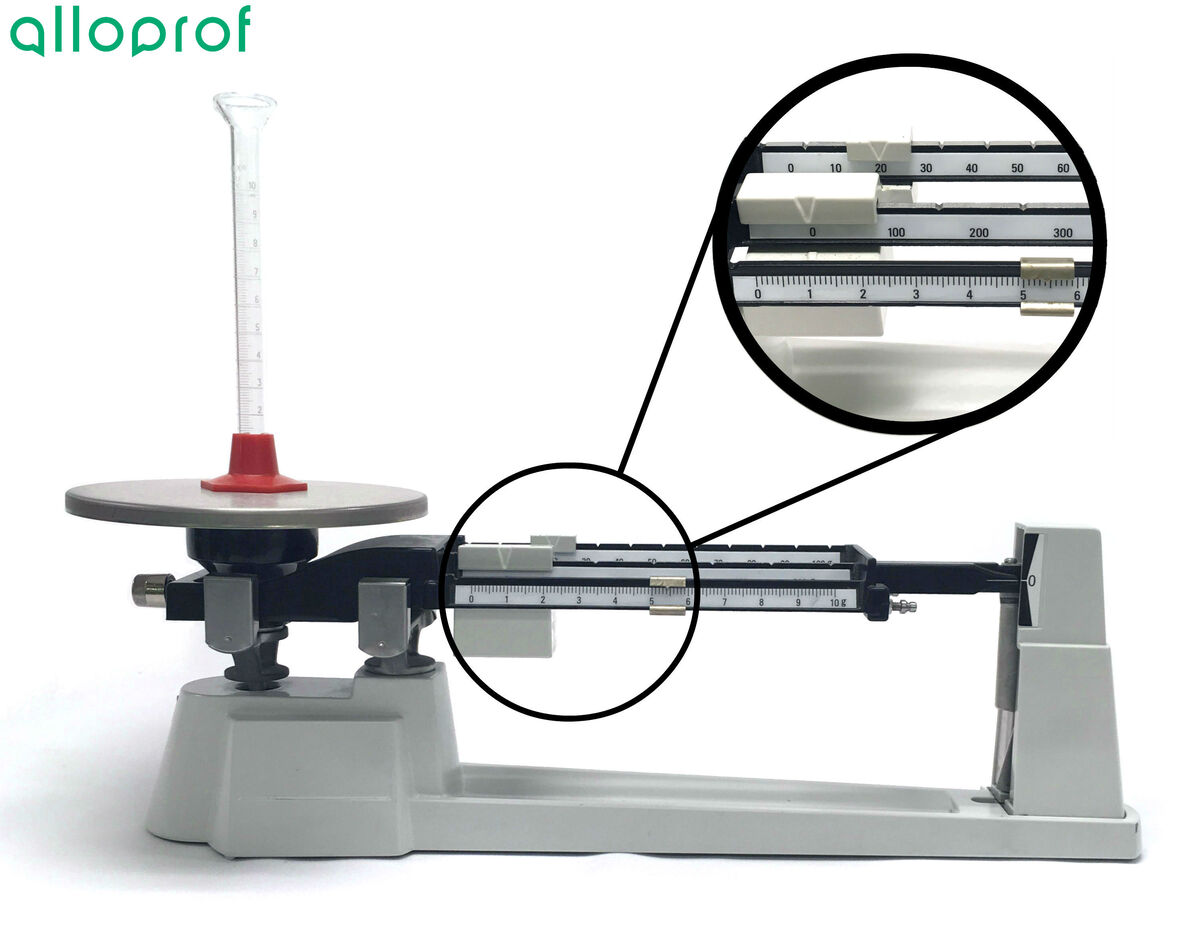
4. Move the rider on the bean with the largest scale until the pointer is lower than zero.

5. Bring the rider one notch to the left so that the pointer is above zero.

6. Repeat steps 4 and 5 with the rider on the second beam.

7. Finally, move the rider on the beam with the smallest scale until the pointer is perfectly aligned with the zero.

Balances are very sensitive to motion. If a person is moving near a balance, it can be difficult to determine the exact mass of an object. It is therefore very important to work calmly in a lab.
8. Add the mass measurements indicated by the riders to find the mass of the object. Record the mass.
9. Reset the riders of the balance to zero.

- Solid to be weighed
- Weighing dish
- Triple beam balance
- Lab coat
- Safety glasses

1. Measure the mass of the weighing dish using the balance. Record it.

2. Place the solid to be weighed in the weighing dish on the pan of the balance.

3. Measure the mass of the weighing dish and the solid. Record it.

4. Calculate the mass of the solid to be weighed.
5. Reset the riders of the balance to zero.

6. Clean and store the equipment.
To determine the mass of a solid, find the difference between the total mass of the solid in the weighing dish (Step 3) and the mass of the empty weighing dish (Step 1).
|{m}_ {{solid}}={m}_ {{weighing\ dish + solid}}-{m}_ {{weighing\ dish}}|
|{m}_{{solid}}:| mass of the solid |(\text {g})|
|{m}_{{weighing\ dish + solid}}:| mass of the weighing dish and the solid |(\text {g})|
|{m}_{{weighing\ dish}}:| mass of the weighing dish |(\text {g})|
The calculated mass represents the mass of the solid. This value does not make it possible to identify with certainty which solid was weighed. However, the mass can be used with the volume to find the density of the object.
The results can be displayed in the form of a table.
Mass of a Solid
| | Solid |
| |m_{weighing\ dish}| | |\text {2.5 g}| |
| |{m}_ {{weighing\ dish + solid}}| | |\text {44.15 g}| |
| |{m}_ {{solid}}| | |\text {41.65 g}| |
- Liquid to be weighed
- Graduated cylinder
- Triple beam balance
- Lab coat
- Safety glasses

1. Measure the mass of an empty graduated cylinder using the balance. Record it.
2. Carefully pour the liquid to be weighed in the graduated cylinder on the pan of the balance.

3. Measure the mass of the liquid and the graduated cylinder. Record it.
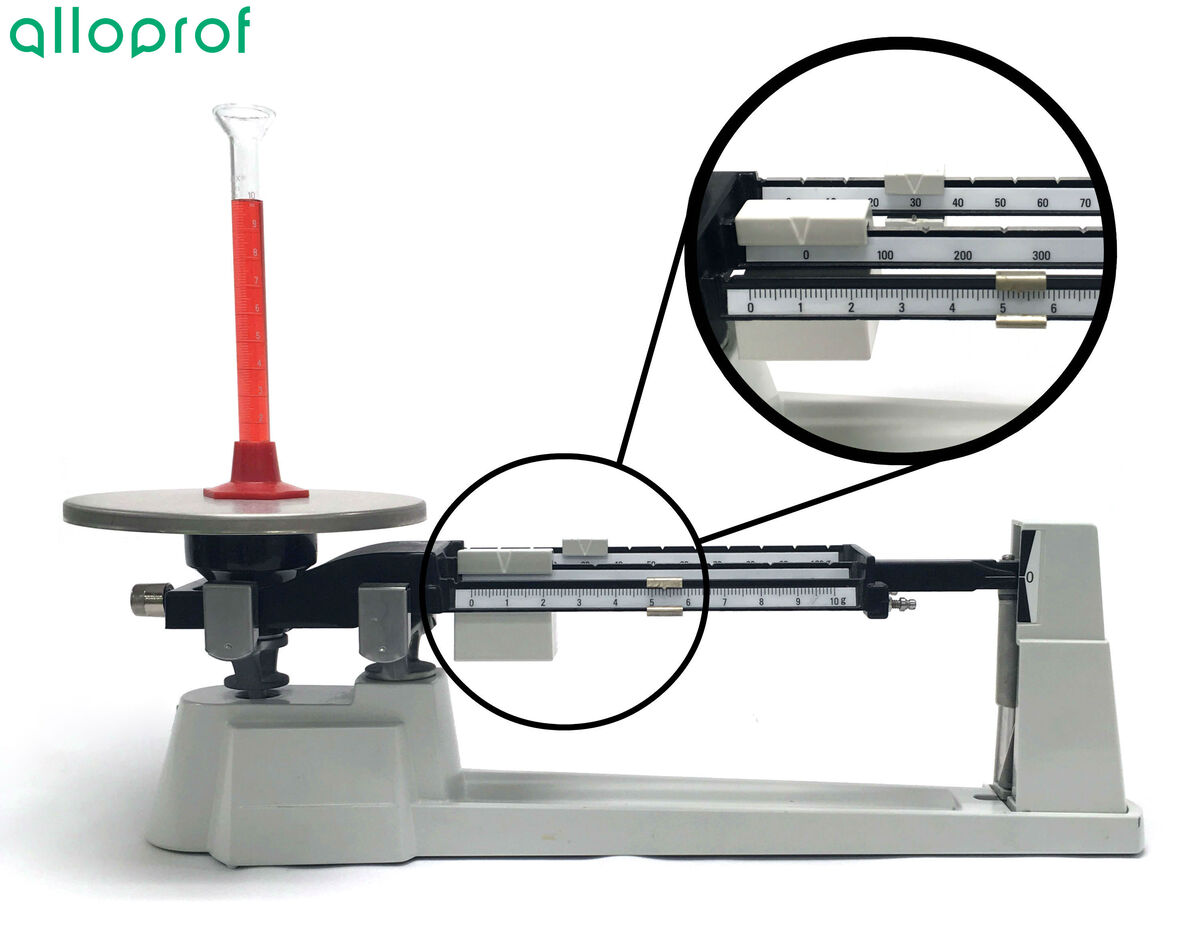
4. Calculate the mass of the liquid to be weighed.
5. Reset the riders of the balance to zero.

6. Clean and store the equipment.
To determine the mass of a liquid, calculate the difference between the total mass of the liquid in the graduated cylinder (Step 3) and the mass of the graduated cylinder (Step 1).
|{m}_ {{liquid}}={m}_ {{graduated\ cylinder + liquid}}-{m}_ {{graduated\ cylinder}}|
|{m}_ {{liquid}}:| mass of the liquid |(\text {g})|
|{m}_ {{graduated\ cylinder + liquid}}:| mass of the graduated cylinder and the liquid |(\text {g})|
|{m}_ {{graduated\ cylinder}}:| mass of the graduated cylinder |(\text {g})|
The calculated mass, or the mass of the liquid, can be used, for example, to determine the density of a substance.
The following results table is an example of a table presenting the results of the experiment:
Mass of a Liquid
| | Liquid |
| |m_{graduated\ cylinder}| | |\text {25.4 g}| |
| |{m}_ {{graduated\ cylinder liquid}}| | |\text {35.35 g}| |
| |{m}_ {{liquid}}| | |\text {9.95 g}| |
- Gas to be weighed
- Syringe
- Triple beam balance
- Lab coat
- Safety glasses

1. Measure the mass of an empty syringe using the balance. Record it.
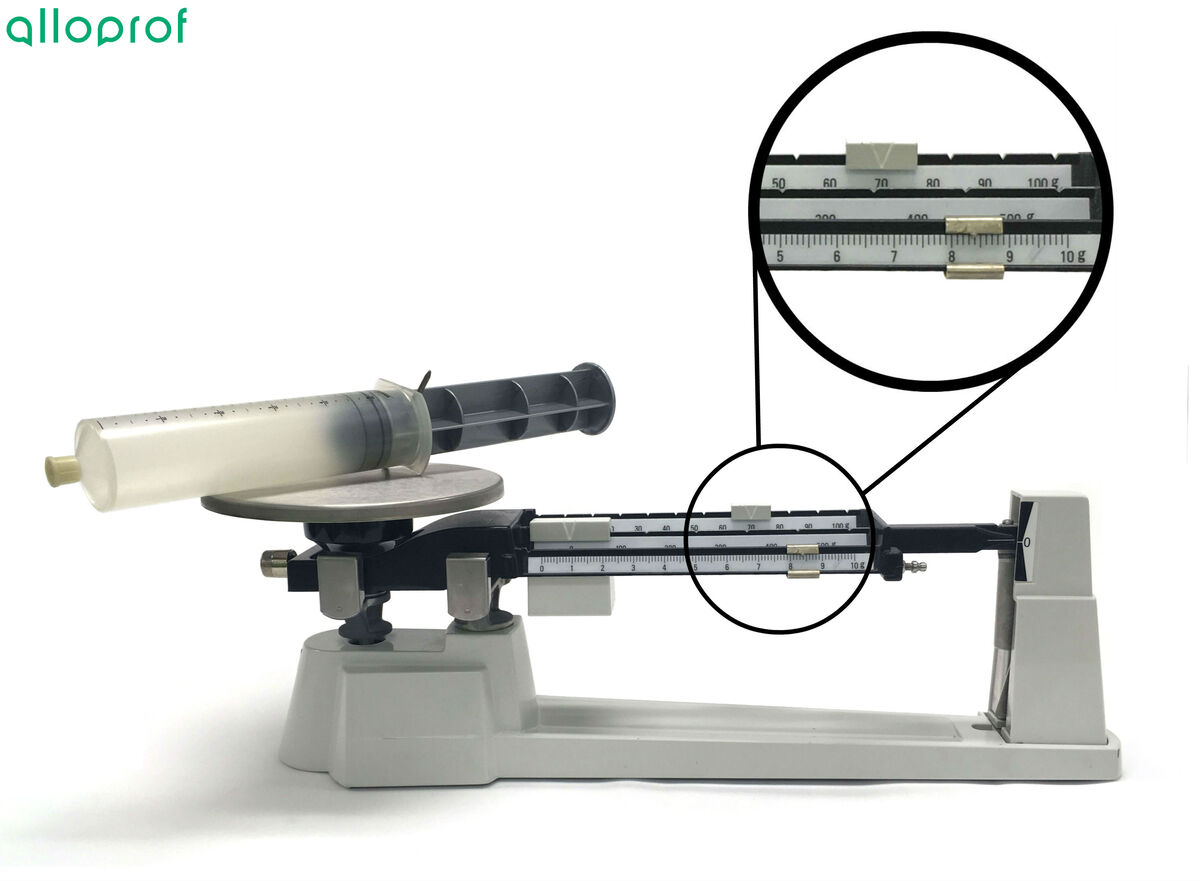
It is important to ensure that the air is completely evacuated from the syringe before placing it on the balance.
Also, it is essential to ensure that the volume of an empty syringe is the same as that of the syringe filled with gas. To achieve this, put the stopper on the syringe and pull the plunger until the plunger hole appears. Inserting a nail into the plunger hole will keep the plunger in place.

2. Fill the syringe with the gas to be weighed.

3. Measure the mass of the gas-filled syringe. Record it.
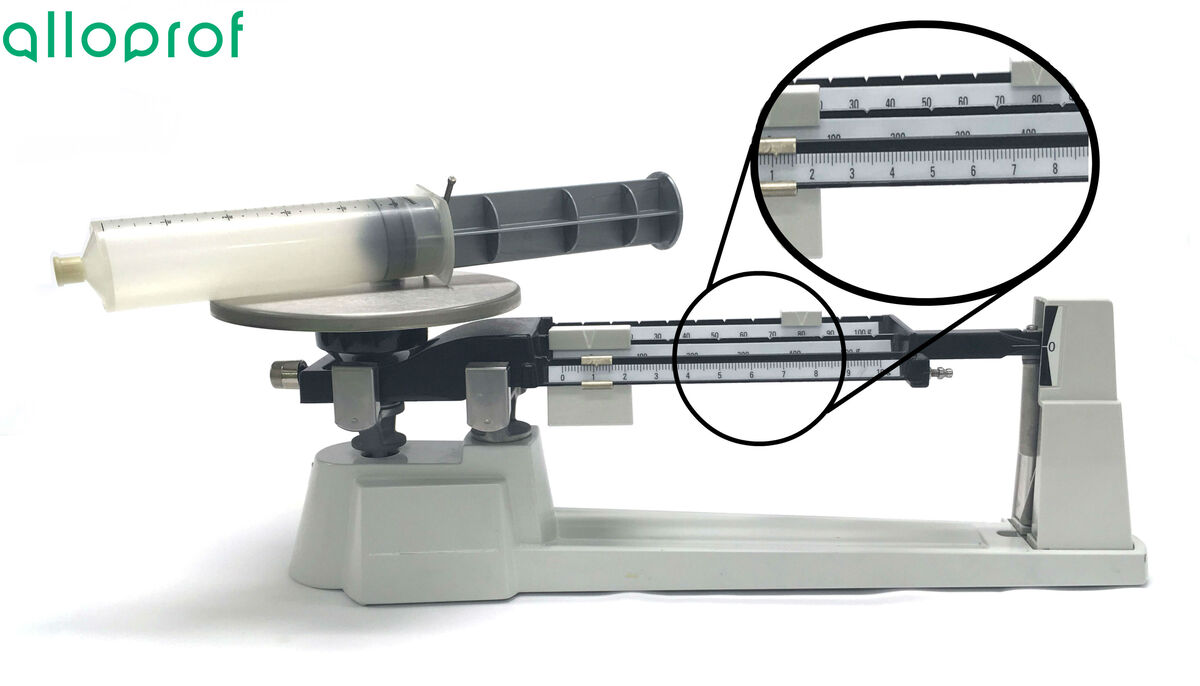
4. Calculate the mass of the gas to be weighed.
5. Reset the riders of the balance to zero.

6. Clean and store the equipment.
To find the mass of the gas, calculate the difference between the total mass of the syringe and the gas (Step 3), and the mass of the empty syringe (Step 1).
|{m}_ {{gas}}={m}_ {{syringe + gas}}-{m}_ {{syringe}}|
|{m}_ {{gas}}:| mass of the gas |(\text {g})|
|{m}_ {{syringe + liquid}}:| mass of the syringe and the gas |(\text {g})|
|{m}_ {{syringe}}:| mass of the empty syringe |(\text {g})|
This procedure, although performed less frequently than those for solids or liquids, is useful when studying the properties of gases.
The following results table is an example of a table showing the results of the experiment.
Mass of a Gas
| | Gas |
| |m_{syringe }| | |\text {78.4 g}| |
| |{m}_ {{syringe + gas}}| | |\text {81.1 g}| |
| |{m}_ {{gas}}| | |\text {2.7 g}| |