This concept sheet explains the procedure to be followed to determine the solubility measurement of a substance. There are different ways of measuring solubility: one technique is shown in this concept sheet.
Solubility is the maximum amount of solute that can be dissolved in a solvent at a given temperature. To determine the solubility, it will therefore be important to control the temperature as much as possible so that it remains constant throughout the experiment.
|
 |
1. Measure 10 mL of distilled water with the graduated cylinder.
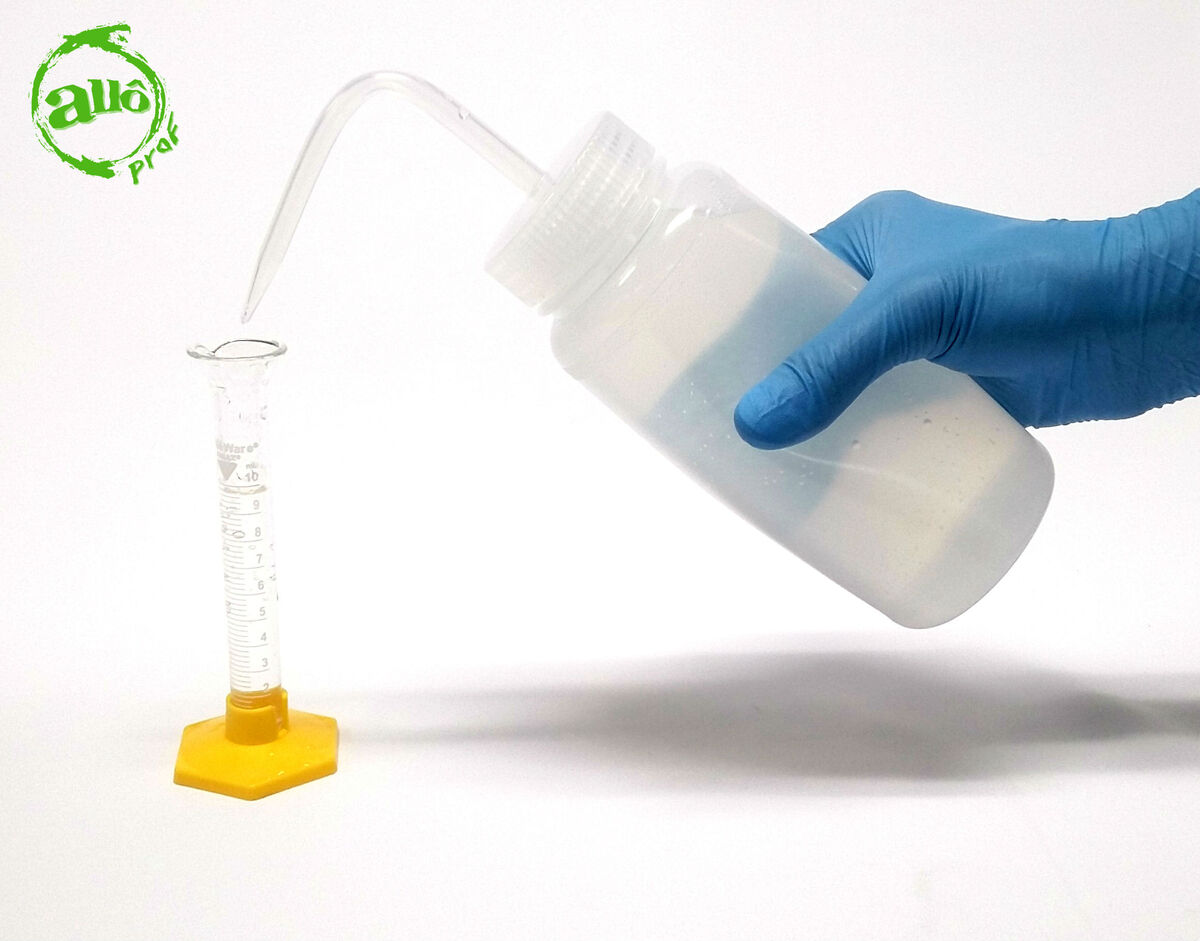
2. Pour the water into the test tube.

3. Measure the temperature of the distilled water in the test tube.

4. Using the balance, weigh the weighing dish with the solid substance and record the result.

5. Using a spatula, add a small amount of the solid substance to the test tube.

6. Place the stopper on the test tube and shake to dissolve the solute completely.
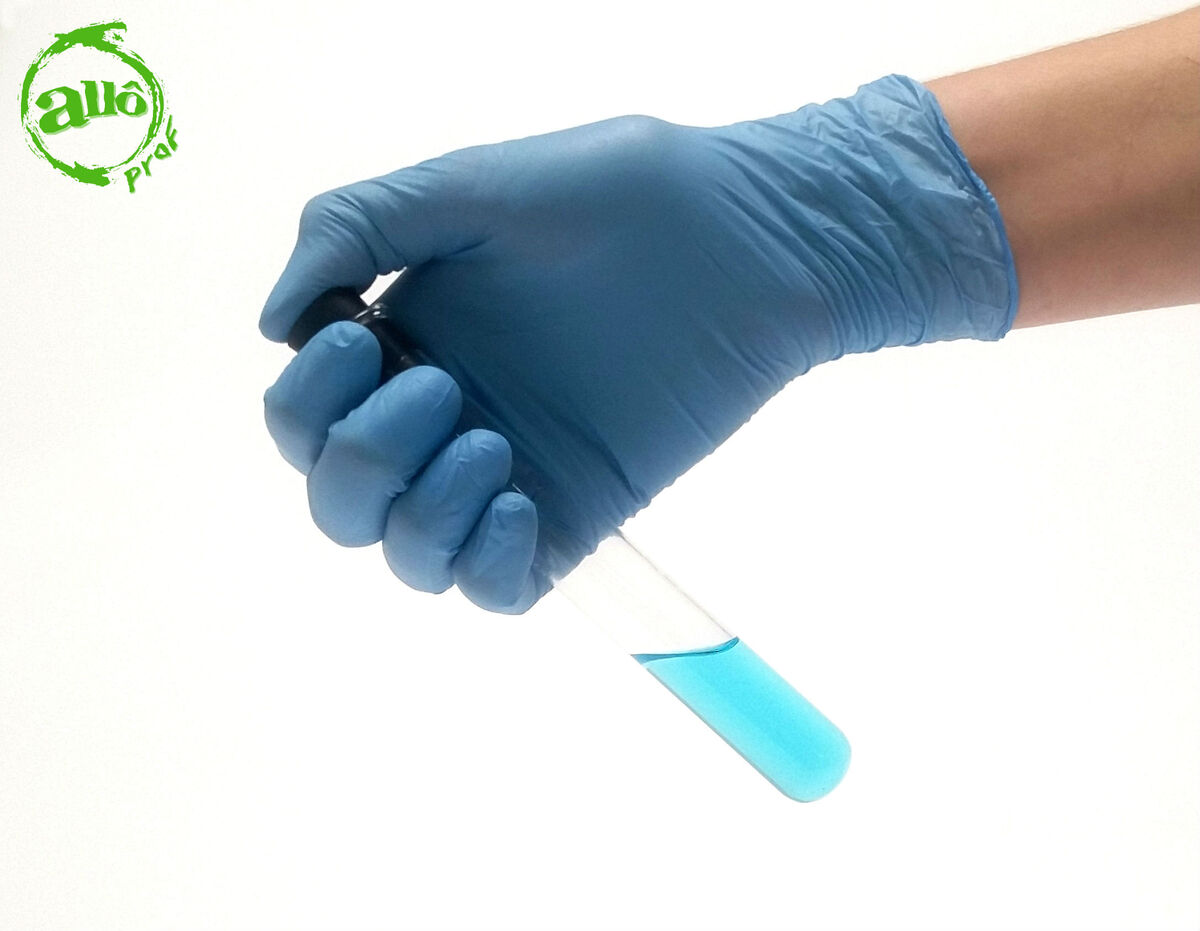
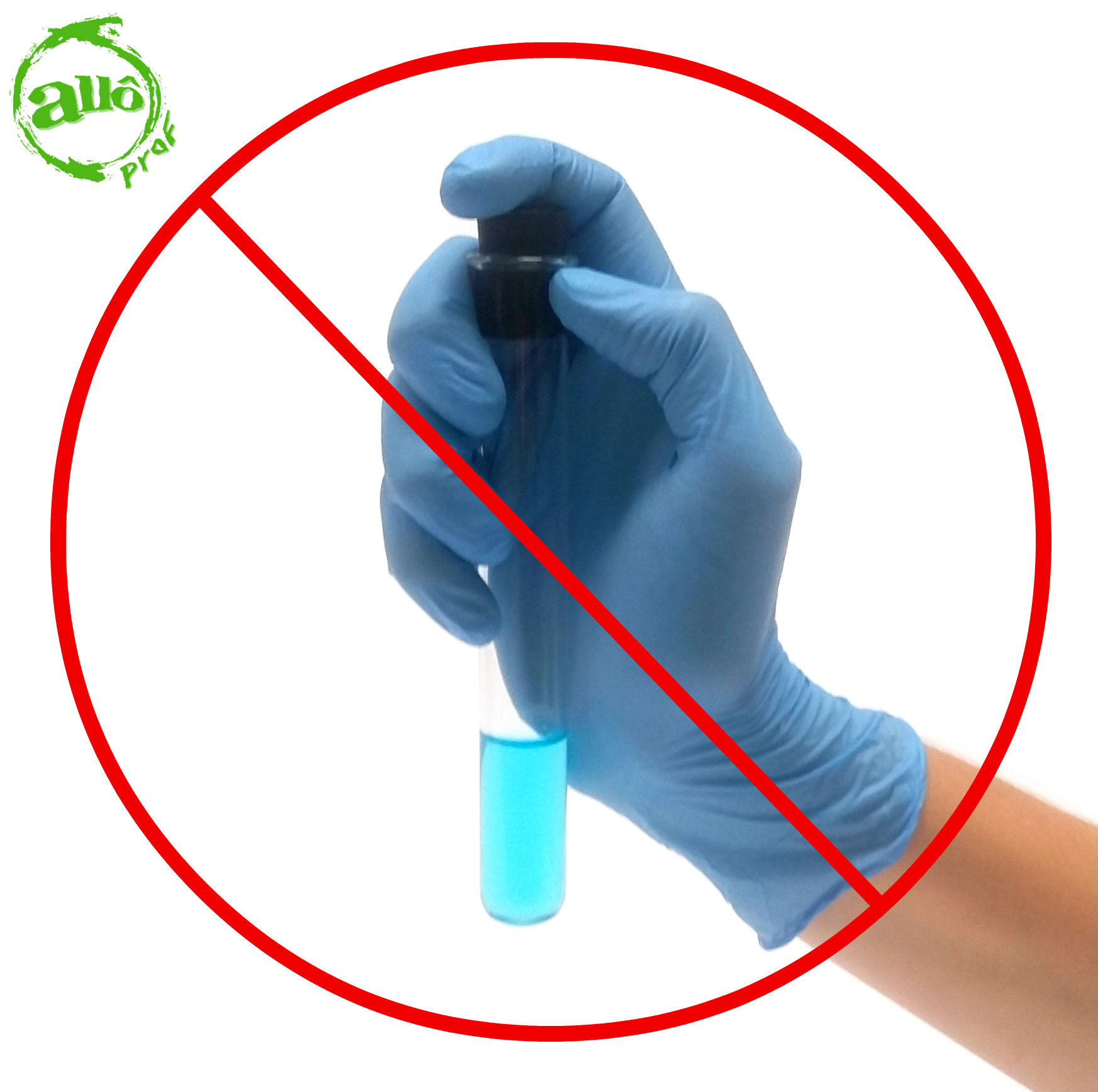
Hands must not be placed on the bottom of the test tube when shaking the test tube. The heat from the human body could be transferred to the mixture and impact the solubility measurement.
In order to prevent the mixture from spilling, it is recommended to hold the test tube by the upper part with the thumb on the stopper. This will ensure a better seal of the stopper on the test tube and will prevent the solution from heating up.
 |
 |
7. If the solute dissolves completely, repeat steps 7 and 8. If the dissolution becomes increasingly slower, add progressively smaller amounts of the solid substance.

The lower the amount of undissolved solid at the end of the procedure, the more accurate the measurement. It is therefore essential to decrease the quantity of the substance to be dissolved as the experiment progresses to obtain the most accurate data possible.
8. If the solid substance no longer dissolves, check the temperature of the solution again.

9. Using the balance, determine the mass of the solid substance remaining in the weighing dish and record the result.

10. Calculate the solubility of the solid substance.
11. Clean the equipment used.
If possible, it is recommended to repeat the experiment a second time to validate the experimental results obtained the first time.
Several data are obtained in the course of the lab.
Calculate the mass of solute that has been dissolved. Since the mass of the weighing dish and the solid substance initially present in the weighing dish has been measured (step 4), and the mass of the weighing dish and the undissolved solid has also been measured (step 9), it is possible to calculate the mass of the dissolved solid substance.
|{m}_{{solid}} = {m}_{{solid(i)}} -{ m}_ {solid(f)}|
where
|{m}_{{solid}}| represents the mass of the dissolved solid substance (in g)
|{m}_{{solid(f)}}| represents the mass of the undissolved solid substance in the weighing dish (in g)
|{m}_ {solid(i)}| represents the mass of the solid substance initially found in the weighing dish (in g)
The solubility can then be calculated. Since solubility is expressed in grams of solute per 100 mL (g/100 mL), our data must therefore be converted to obtain an equivalent rate.
|\text {Solubility} = \displaystyle \frac {{m}_ {solid} \times 100} {{V}_ {{solvent}}}|
where
|{m}_ \text{solid}| represents the mass of the dissolved solid substance (in g)
|{V}_ {\text{solvent}}| represents the volume of the solvent used (in mL)
When all calculations have been completed, the results should be presented in a results table. Here is an example of a table template that can be used.
Solubility of the Solid Substance
| | Solid substance |
| |{T}_{{i}}| | ºC |
| |{m}_{{solid(i)}}| | g |
| |{m}_{{solid(f)}}| | g |
| |{m}_{{solid}}| | g |
| |{V}_{{solvent}}| | mL |
| |{T}_{{f}}| | ºC |
| |\text {Solubility}| | g/100 mL |
When the solubility has been determined, the solid substance can be identified. Using reference tools or manuals, a comparison between the experimental data and those displayed in various tables can help determine the solid substance.
If the solid substance is already known, it is possible to compare the value obtained experimentally with the known value.
Be sure to verify the solubility with the temperature in mind, since it influences the amount of solute that can be dissolved. If the temperature has changed during the experiment, there could be a difference between the experimental data and that presented in the reference tables.