Solubility is a characteristic physical property. It is the maximum amount of solute that can be dissolved in a given volume of solvent.
Solubility is measured in grams per hundred millilitres |(\text{g/100 mL})| or grams per litre |(\text{g/L})|. The temperature when the solubility was measured is also mentioned, because it affects solubility.
To calculate the solubility in |\text{g/100 mL}|, use the following formula.
||s= \dfrac{m\times100}{V}||
where
|s| is the solubility |(\text{g/100 mL})|
|m| is the maximum mass of the solute |(\text{g})|
|V| is the volume of the solvent |(\text{mL})|
A volume of |125\ \text{mL}| of water is used to make a maximum of |12.0\ \text{g}| of sodium bicarbonate |(\text{NaHCO}_3)| soluble at |20^\circ\text{C}|. What is the solubility of the solute in |\text{g/100 mL}|?
Start by listing the given values in the problem.
|\begin{align}V&=125\ \text{mL} &m=12.0\ \text{g}\\ s&=? \end{align}|
Knowing that |s= \dfrac{m\times100}{V}| when |s| is in |\text{g/100 mL}|:
|\begin{align}s&=\dfrac{12.0\ \text{g} \times100}{125\ \text{mL}}\\s&\approx9.60\ \text{g}/100\ \text{mL} \end{align}|
So, the solubility of sodium bicarbonate |(\text{NaHCO}_3)| in the water is |9.60\ \text{g}/100\ \text{mL}| at |20^\circ\text{C}.|
The solubility of sodium chloride |(\text{NaCl})| in the water is |36.0\ \text{g}/100\ \text{mL}| at |25^\circ\text{C}.| What is the minimum amount of water needed to dissolve |150\ \text{g}| of sodium chloride?
Start by listing the given values in the problem.
|\begin{align}s&=36.0\ \text{g}/100\ \text{mL} &m=150\ \text{g}\\ V&=? \end{align}|
If |s= \dfrac{m\times100}{V}| when |s| is in |\text{g/100 mL}|, then |V= \dfrac{m\times100}{s}:|
|\begin{align}V&=\dfrac{150\ \text{g} \times 100}{36.0\ \text{g}/100\ \text{mL}}\\V&\approx417\ \text{mL} \end{align}|
So, it will take at least |417\ \text{mL}| of water to dissolve |150\ \text{g}| of sodium chloride |(\text{NaCl})| at |25^\circ\text{C}.|
Do not confuse solubility with concentration. Solubility is a characteristic physical property, while concentration is a non-characteristic physical property of a solution.
Also, solubility refers to the maximum amount of solute that can be dissolved in a volume of solvent. However, concentration refers to the amount of solute dissolved for a total volume of solution.
When preparing a solution, it is important to know the solubility of the solute to obtain the desired mixture. In fact, if we try to dissolve too much solute in a solvent, only part of the solute will be dissolved and the rest of the solute will settle at the bottom of the container. So, when the solubility limit is reached, the solution is said to be saturated. When the solubility limit is surpassed, the excess solute does not dissolve. This creates a supersaturated solution.
The solubility of sucrose, a type of sugar, is approximately |190\ \text{g/100 mL}| in water at |25^\circ\text{C}|.
|
Unsaturated solution of sucrose |
Supersaturated solution of sucrose |
|---|---|
|
|
|
|
Here, |100\ \text{g}| of sucrose is dissolved in |100\ \text{mL}| of water. The amount of solute does not exceed its solubility. The mixture is homogeneous. |
Here, |250\ \text{g}| of sucrose is mixed with |100\ \text{mL}| of water. The amount of solute exceeds its solubility. There is still undissolved solute at the bottom of the glass. The mixture is heterogeneous. |
Multiple factors can have an impact on the solubility of a solute, for example: the nature of the solute or solvent, the solution’s temperature, the pressure, etc.
Not all solutes have the same solubility. Oxygen, which is a gas, is not very soluble in water. Conversely, sodium chloride, a solid salt, is very soluble in water. However, aluminum is a solid that is not very soluble in water.
Furthermore, solvents do not all have the same ability to dissolve a solute. Sodium chloride (table salt) is highly soluble in water, but slightly soluble in ethanol.
The following table highlights the difference in solubility of various solutes in water at |20^\circ\text{C}.|
|
Solute |
Solubility in water (|\text{g/100 mL}| at |20\ °\text{C}|) |
|---|---|
|
Oxygen gas |(\text{O}_2)| |
|0.001| |
|
Sodium chloride |(\text{NaCl})| |
|35.7| |
|
Sodium hydroxide |(\text{NaOH})| |
|111.1| |
|
Glucose |(\text{C}_6\text{H}_{12}\text{O}_6)| |
|100.0| |
|
Ethanol |(\text{C}_2\text{H}_6\text{O})| |
Mixes in any proportion |
Temperature has a large impact on the solubility of solutes. Generally, the solubility of liquid and solid solutes increases as the temperature of the solution increases. A greater agitation of solvent particles promotes mixing of solute particles.
In the case of gaseous solutes, an increase in temperature decreases their solubility in a liquid solvent. In this case, the gas molecules are too agitated to mix adequately with a liquid. An increase in temperature increases the agitation of the gas particles, which is already excessive, causing the gas particles to flow out of the solution.
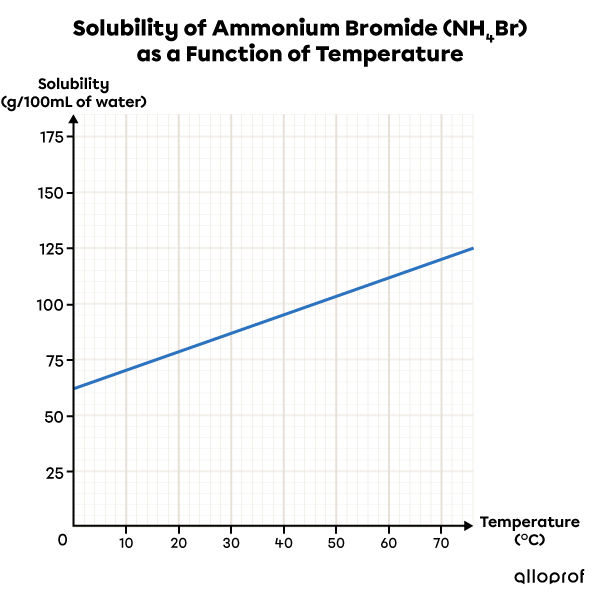
This solubility graph shows that as the temperature increases, the solubility of ammonium bromide |(\text{NH}_4\text{Br})| in water increases. So, warm water is a better solvent than cold water for this solute.
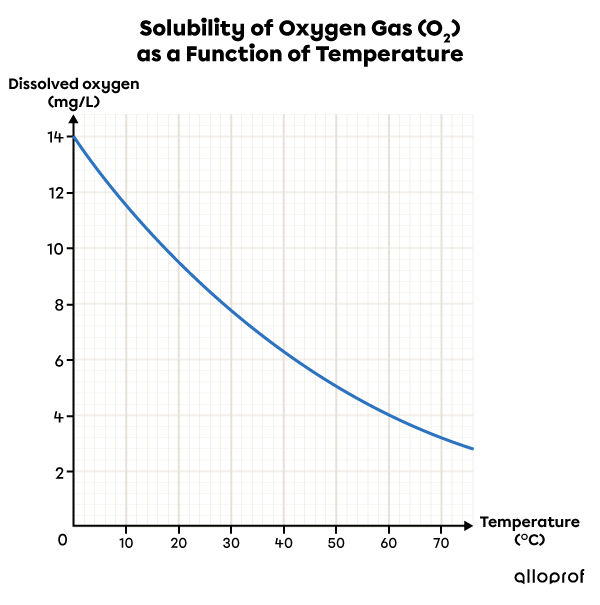
Source des données : Watterman, Adami, 2005.
This solubility graph shows that as the temperature increases, the solubility of oxygen gas |(\text{O}_2)| in water decreases. So, cold water is a better solvent than hot water for this solute.
Watterman, K.; Adami, R. (2005). Accelerated aging: Prediction of chemical stability of pharmaceuticals. International Journal of Pharmaceutics, 293(1-2), 101-125. https://www.sciencedirect.com/science/article/abs/pii/S0378517305000104…

