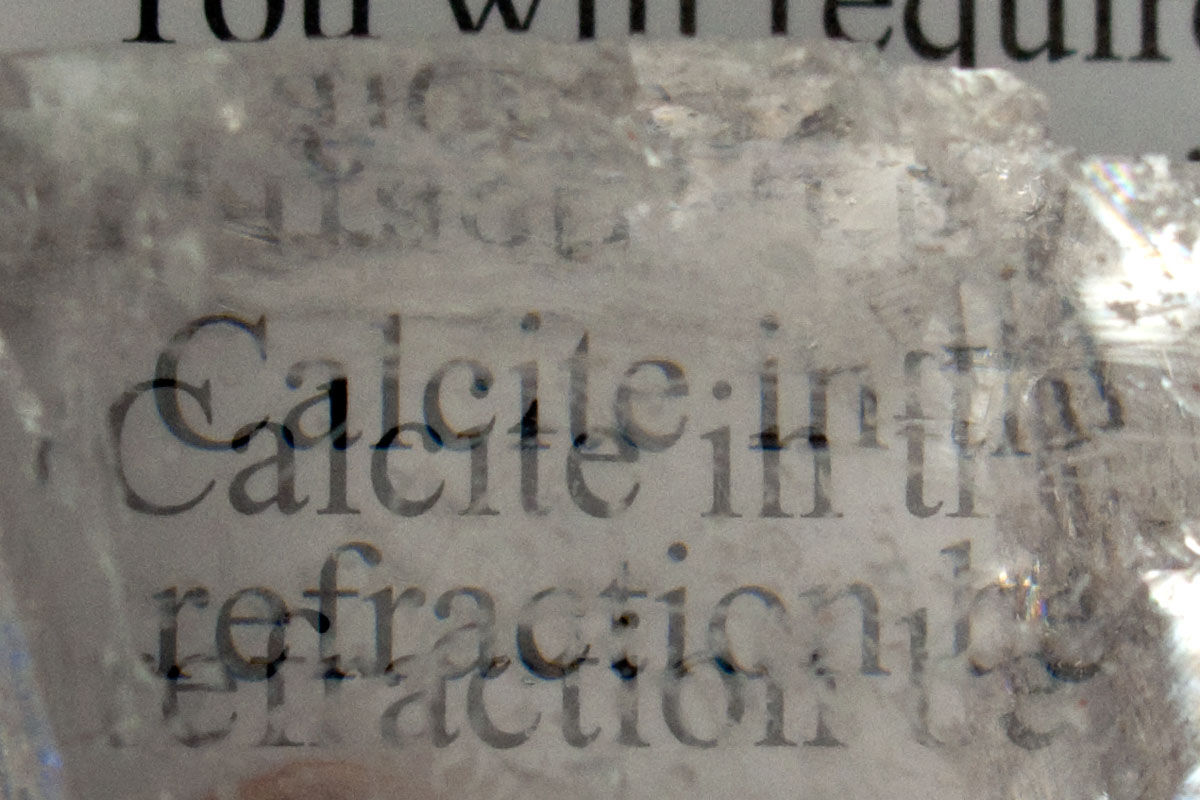A mineral is a natural inorganic substance, although sometimes of organic origin, which is distinguished from another type of mineral by its chemical composition. Each mineral is an arrangement of atoms according to a particular symmetry, thus forming a given crystal lattice (a single type of crystal).
There are over 4 000 different types of minerals. All minerals are usually found in a solid state, although they can be in a liquid state if subjected to high temperatures and pressures.
Unlike a rock, a mineral is a pure substance made up of identical elements. It is thus possible to identify minerals since they have specific properties. In addition, a mineral has only one dominant colour, although impurities are often found in samples.
Minerals and ores should not be mistaken for each other. An ore is a rock extracted from the lithosphere which contains a quantity of mineral large enough to justify its exploitation. When ores are found, mines are built to exploit the vein to its maximum capacity.
Luster
The luster of a mineral represents the way in which light is reflected off the mineral’s surface.
The luster can be metallic (if it has a highly reflective or shiny surface), sub-metallic (if the surface is slightly reflective), or non-metallic (if the surface is not reflective).
Check a mineral’s luster by placing the sample under a light source and observe the mineral’s reflection.
Gold exhibits a metallic luster, while quartz has a non-metallic, glass-like luster.
Colour of the Mineral
The colour of the mineral is its predominant colour without taking into account impurities.
To check the colour of the mineral, it is preferable (if possible) to break the mineral to note the observable colours on the breakage.
Pyrite has a golden colour, while galena is grey.
The colour of a mineral does not always identify a mineral. In fact, we define the colour in two ways:
-
idiochromatic mineral: the colour is invariable and characteristic, it is therefore used to identify the mineral;
-
allochromatic mineral: the colour is variable and depends on the impurities found in the mineral.
Malachite (left) is an example of an idiochromatic mineral while fluorite (right) is an allochromatic mineral.
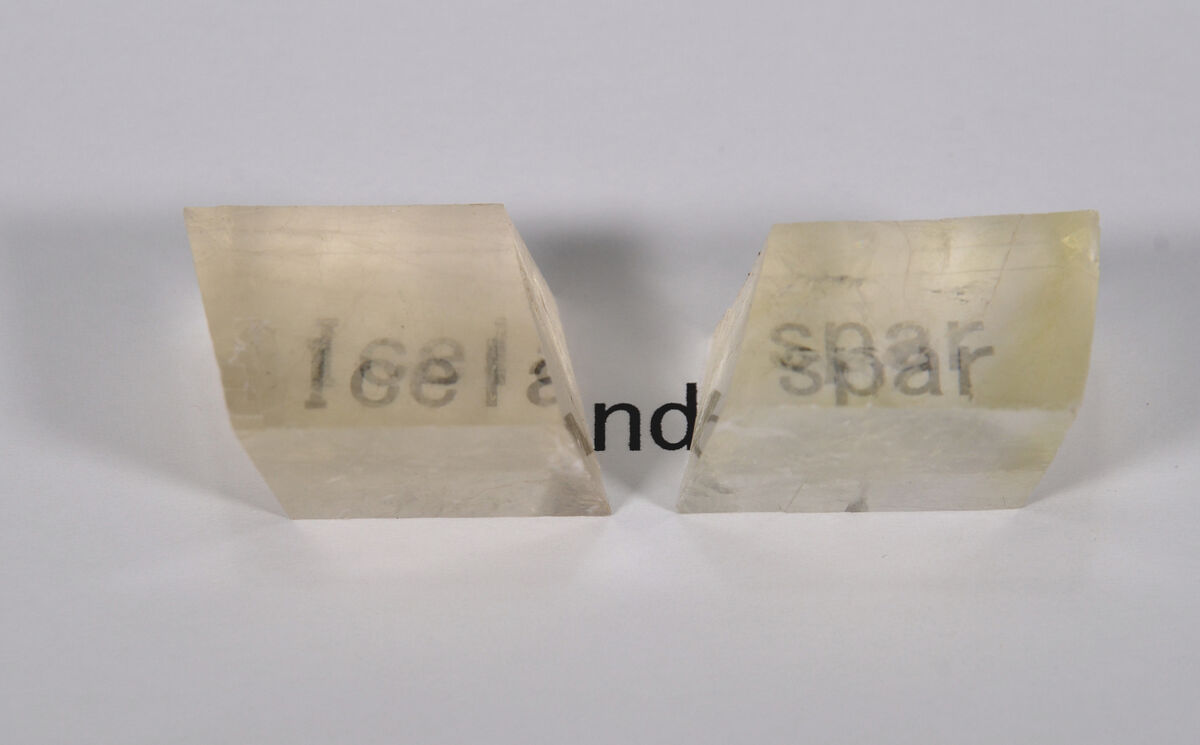
Transparency and Opacity
Transparency is the property of minerals to transmit incident light.
The more translucent a mineral, the more light it lets pass through — although objects can appear blurry when looking through the mineral.
To observe the transparency of a mineral, it is necessary to observe an object through a mineral and determine how blurry the observed object is or not.
Iceland spar (a variety of calcite) is transparent, while apatite is opaque.
Refractive Index
The refractive index is a material property that describes how the material affects the speed of light travelling through it.
Iceland spar, a form of calcite, is a mineral that has the particularity of causing double refraction. Light is deviated in two ways as it passes through this mineral. It therefore produces the image in double.
Kim Christensen, Shutterstock.com
Streak Colour
The streak colour is the colour of the trace left by a mineral after being rubbed on an unglazed porcelain plate.
To determine the colour of the streak, rub the mineral on an unglazed porcelain plate and note the observed colour.
Pyrite (left) produces a brownish-black streak while rhodochrosite produces a white streak.
Reaction to Acid
Effervescence is the ability of a mineral to react in the presence of an acid by producing bubbles (gas).
These bubbles are caused by the release of a gas produced by the chemical reaction of the mineral and the acid. This phenomenon is seen in minerals composed of carbonates.
To check whether the mineral is effervescent, just place one drop or two of hydrochloric acid on the mineral and observe if bubbles appear.
Calcite produces effervescence when acid is placed on its surface.
Other chemical properties that can be tested in the laboratory include:
-
photoluminescence, which is the ability of certain minerals to transform chemical energy into light;
-
colouring under flame, which is the reaction of certain minerals to the heat of a flame by emitting light of a given colour. The colour produced is characteristic of the chemical composition of the mineral;
-
chemical composition, used to determine the grouping of chemical elements presented by means of a chemical formula;
-
solubility in water, the property of the mineral to dissolve or not in water.
Hardness
Hardness is the resistance of the mineral to scratches.
Some minerals can be softer, while others are much harder. To check hardness, scratch a mineral with a fingernail, nail, and steel file (or knife) and determine which objects can scratch the mineral.
To classify their minerals according to their hardness, we use the Mohs scale.
|
Hardness |
Hardness test |
Mineral |
|
|---|---|---|---|
|
1 |
Mineral breaks down under a fingernail |
Talc |
|
|
2 |
Mineral scratchable by a fingernail |
Gypsum |
|
|
3 |
Mineral scratchable by a penny |
Calcite |
|
|
4 |
Mineral lightly scratchable by a knife |
Fluorite |
|
|
5 |
Mineral scratchable by a knife |
Apatite |
|
|
6 |
Mineral scratchable by a file |
Feldspar |
|
|
7 |
Mineral that can scratch glass |
Quartz |
|
|
8 |
Mineral scratchable by tungsten |
Topaz |
|
|
9 |
Mineral scratchable by silicon |
Corundum |
|
|
10 |
Mineral scratchable by another diamond |
Diamond |
|
Cleavage and Fracture
Cleavage is the property of certain minerals to break down, forming flat, smooth surfaces.
Fracture is the property of some minerals to break irregularly in all directions, without a flat surface.
To study cleavage, it is necessary to subject a mineral to an impact and analyze the breakage in the crystal structure.
Fluorite exhibits a characteristic cleavage.
Density
Density is the characteristic property that represents the ratio between the amount of matter of a mineral and its volume.
The density of gold is |19.3| g/ml, while the density of fluorite is |3.2| g/ml.
Magnetism
Magnetism is the property that certain minerals have of attracting a magnet.
To test the magnetism of a mineral, it is necessary to approach a magnet to a mineral and check whether there is attraction or repulsion between the mineral and the magnet.
Magnetite has magnetic properties, while talc has no such properties.
Other physical properties can be used to identify a mineral:
-
relative density is the comparison of the density of a mineral to the density of quartz (which is approximately |2.7| g/ml);
-
touch is the sensation experienced when running one’s hand over the mineral (rough, soft, etc.).
Metallic minerals are made up of metallic elements. These can be melted down to obtain new products. Metallic minerals of metal origin represent more than half of all mineral resources in Quebec.
Metallic minerals have certain characteristics:
-
they are generally related to igneous rocks;
-
they are usually hard and shiny or have their own luster;
-
they are ductile and malleable.
Here are metallic minerals from which some metals are extracted.
|
Metallic minerals |
Metals extracted |
Examples of usage |
|---|---|---|
|
Chalcopyrite |
Copper and gold |
Plumbing pipes, jewelry, coins |
|
Hematite |
Iron |
Steel and cast iron alloys in construction |
|
Magnetite |
||
|
Sphalerite |
Zinc |
Galvanization of steels (rust protection) |
Industrial minerals represent nearly |30|% of all mineral rocks in Quebec. These minerals are non-metallic and are extracted from rocks to be used for industrial purposes.
Here are some examples of these extractions.
|
Industrial materials |
Industrial minerals |
Examples of usage |
|---|---|---|
|
Serpentinite |
Asbestos (chrysotile) |
Insulation (space shuttle, fire protection suit, house, etc.) |
|
Marble |
Graphite |
Lead pencil, alloy composite for sports (snowshoes, poles, skis, etc.) |
|
Quartzite |
Quartz |
Water purification, decoration |
Only four types of gemstones can be found in the world. However, when visiting a jewelry store or a boutique selling collectible minerals, there seems to be an endless number of them.
However, to earn the title of gemstone, a mineral must have the following characteristics:
-
according to the Mohs scale, the hardness of a gemstone should range between 7.5 and 10;
-
only one crystal is found in the gemstone;
-
the gemstone must be aesthetically beautiful with respect to its colour and must be rare.
Gemstones are weighed in carats. One carat is equivalent to |200| mg of the mineral.
Here is a table that summarizes the main properties of the four gemstones available in the world.
|
Name of gemstone |
Colour |
Hardness (according to the Mohs scale) |
Chemical composition |
Usages |
|---|---|---|---|---|
|
Diamond 
|
Transparent |
10 |
Carbon |(C)| |
Jewelry, ophthalmic scalpels, electrodes |
|
Sapphire 
|
Blue |
9 |
Aluminum oxide |(Al_{2}O_{3})| |
Jewelry |
|
Ruby 
|
Red |
9 |
Aluminum oxide |(Al_{2}O_{3})| |
Jewelry, watchmaking, lasers |
|
Emerald 
|
Green |
7.5 |
Aluminum and beryllium silicate |(Be_{3}Al_{2}(Si_{6}O_{18}))| |
Jewelry |
As can be seen, gold, garnet, topaz, quartz, amethyst, and turquoise are not gemstones. In fact, gold is a metal. Metals, like gold and silver, are not gemstones. As for other stones, they belong to the semi-precious stones category.
To be considered a semi-precious stone, the mineral must be quite hard and coarse; it must be transparent and of a beautiful hue. Finally, it must be relatively rare... or trendy.

With the development of science, humans have observed that certain elements of nature help plants to grow. However, on a large scale, these minerals can be devastating when spread as fertilizer in large quantities on farmlands. Plants are unable to absorb all these fertilizers. The excess is washed away by rainwater which carries it to rivers and lakes. This creates bioaccumulation and, in the short term, eutrophication of waterways.
Once the exploitation of a mine is finished, some companies leave the mine open. Scientists have noticed that these mines are a potential source of contamination. Indeed, the water that falls in these wells reacts with the minerals because it is an excellent solvent. By infiltration, this water travels in underground rivers until it reaches a water table. This accumulation of minerals leads, in the long term, to contamination of the water table. As a result, the surrounding populations can no longer use this water table, because it is unfit for consumption.
It should also be considered that for the construction of mines and mining, large amounts of water are used. This water, in contact with minerals, may be unfit for consumption.
Other major environmental impacts from mining or mineral processing are also likely to occur.
-
There may be, in the medium or long term, a depletion of resources, since these minerals are not renewable resources.
-
Large wooded areas can be destroyed when mines are developed, since roads must be built to access the mines, and factories are built nearby. In addition, the passage of heavy machinery over the ground compacts the soil.
-
Greenhouse gases (GHGs) are emitted by the use of machinery in mines. In addition, heavy machinery also generates noise pollution.
-
Wildlife will be displaced to other territories, driven away by humans exploiting mineral resources.