A pure substance is a substance that consists of only one type of particle.
In the following examples, all the particles of each substance are identical. Therefore, they are pure substances.
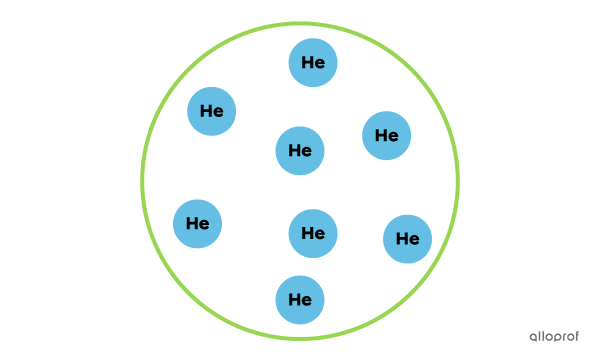
This is a pure substance, because it contains only helium atoms.
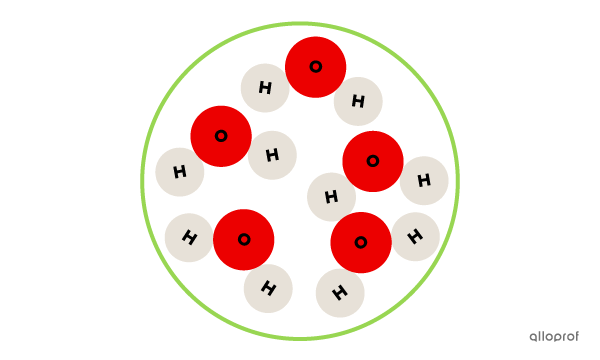
This is a pure substance, because it contains only water molecules.
Pure substances include elements and compounds.
An element is a pure substance that consists of one type of atom.
An element can be a single atom or a molecule made up of several identical atoms. All types of atoms are listed and classified in the periodic table of elements.
An oxygen atom |(\text{O}),| an oxygen molecule |(\text{O}_2)| and an ozone molecule |(\text{O}_3)| are all three elements, because they contain only one type of atom–the oxygen atom |(\text{O}).|



It is possible to tell whether a substance is an element by its chemical formula. When the chemical formula contains a single capital letter, the substance is an element. This is because the chemical symbol for each element in the periodic table always contains a single capital letter.
The number of kinds of atoms in a substance is the same as the number of capital letters in its chemical formula.
-
An atom of bromine |({\color{#EC0000}{\text{B}}\text{r}})| is an element, because it contains only bromine |(\text{Br}).|
-
A molecule of chlorine |({\color{#EC0000}{\text{C}}\text{l}_2})| is an element, because it contains only chlorine |(\text{Cl}).|
-
A molecule of ozone |({\color{#EC0000}{\text{O}}_3})| is an element, because it contains only oxygen |(\text{O}).|
Each of these chemical formulas contains only one capital letter.
A compound is a pure substance composed of several types of atoms.
A compound is always made up of at least two atoms of different types. All types of atoms are listed and classified in the periodic table of elements.
Sodium chloride |(\text{NaCl}),| methane |(\text{CH}_4)| and nitric acid |(\text{HNO}_3)| are compounds because each of them consists of several types of atoms.

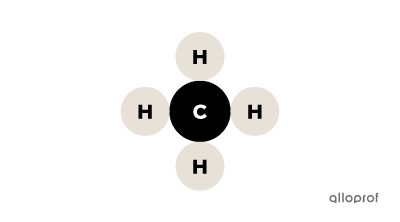
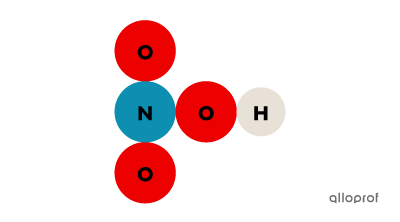
It is possible to tell whether a substance is a compound by its chemical formula. When the chemical formula contains more than one capital letter, the substance is a compound. Since the chemical symbol for each element in the periodic table always contains a single capital letter, multiple capital letters in the chemical formula of a substance indicate the presence of multiple atoms.
The number of kinds of atoms in a substance is the same as the number of capital letters in its chemical formula.
-
Hydrochloric acid |({\color{#EC0000}{\text{HC}}\text{l}})| is a compound, because it contains two types of atoms: hydrogen |({\color{#EC0000}{\text{H}}})| and chlorine |({\color{#EC0000}{\text{C}}}\text{l}).|
-
Glucose |({\color{#EC0000}{\text{C}}_6}{\color{#EC0000}{\text{H}}_{12}}{\color{#EC0000}{\text{O}}_6})| is a compound, because it contains three types of atoms: carbon|({\color{#EC0000}{\text{C}}}),| hydrogen |({\color{#EC0000}{\text{H}}})| and oxygen|({\color{#EC0000}{\text{O}}}).|
-
Water |({\color{#EC0000}{\text{H}}_2}{\color{#EC0000}{\text{O}}})| is a compound, because it contains two types of atoms: hydrogen |({\color{#EC0000}{\text{H}}})| and oxygen |({\color{#EC0000}{\text{O}}}).|
Each of these chemical formulas contains more than one capital letter.
Une erreur s’est glissée dans cette vidéo.
À 1 min 49 s, la formule chimique est plutôt |\text{HCl},| et non |\text{HCl}_2|.