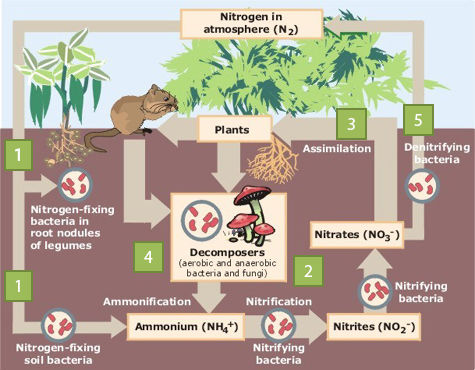
The nitrogen cycle is a biogeochemical cycle that involves all nitrogen exchanges on the planet.
Nitrogen is the most abundant atmospheric gas (air contains 78% of this gas). Nitrogen is essential for the functioning of living organisms. It is used in particular to manufacture proteins and to produce nitrogenous bases present in DNA. However, it cannot be absorbed directly in this form by most living organisms. Bacteria are responsible for transforming nitrogen from the atmosphere into a form that can be assimilated by other living organisms. It is through its biogeochemical cycle that nitrogen can pass from one form to another.
It is worth noting that the nitrogen cycle processes take place both in the lithosphere and in the hydrosphere.
The main processes that take place during the nitrogen cycle are:
Some bacteria, living in the soil or in water, capture atmospheric nitrogen and transform it into nitrogen that can be used by plants and animals, namely ammonia |\small(NH_{3})|. A portion of ammonia is used by plants and animals, while another portion reacts with hydrogen to form ammonium |\small{(NH_{4}}^{+})|. Bacteria capable of fixing nitrogen include cyanobacteria and certain bacteria, such as those of the genus Rhizobium, living in symbiosis with plants (including legumes).
Bacteria oxidize ammonium |\small{(NH_{4}}^{+})| to form nitrites |\small{(NO_{2}}^{-})| and other bacteria oxidize nitrites |\small{(NO_{2}}^{-})| to form nitrates |\small{(NO_{3}}^{-})|. These are two oxidation reactions.
Plants are able to absorb nitrate and ammonium present in the soil or in water through their roots. Plants are the only primary source of nitrogen available to herbivorous animals. Herbivorous animals ingest their nitrogen by eating plants. Nitrogen then follows the food chain. Carnivores ingest their nitrogen by feeding on herbivorous animals or other animals.
Nitrogen is found in plant and animal waste (urine, stools, dead organisms, etc.). Some fungi and bacteria break down these substances to produce ammonia. This ammonia will dissolve to form ammonium.
Bacteria called denitrifiers convert nitrates into nitrogen gas. The nitrogen then returns to the atmosphere. This chemical reaction also produces carbon dioxide |\small(CO_{2})| and nitrogen oxide |\small(N_{2}O)|.
Some of the natural factors that can alter the nitrogen cycle include temperature, humidity, and pH. However, with the explanations given above, it is understood that human activity is unfortunately the factor that has the most impact on the change in the nitrogen cycle. The fertilizers that we spread are rich in ammonia |\small(NH_{3})|, in ammonium |\small{(NH_{4}}^{+})|, and in nitrates |\small{(NO_{3}}^{-})|. Through leaching, this surplus of nitrogen compounds ends up in waterways.
The use of fossil fuels in engines and thermal power plants transform nitrogen into nitrogen oxide. This increases denitrification. However, this phenomenon also releases a small amount of nitrogen oxide into the atmosphere. |\small(N_{2}O)|. Nitrogen oxide is a greenhouse gas that contributes to the destruction of the ozone layer in the stratosphere. It is important to know that one molecule of |\small N_{2}O| is 200 times more effective than one molecule of |\small CO_{2}| at creating a greenhouse effect.