The atmosphere refers to the gaseous envelope surrounding a celestial body (star, planet, natural satellite).
Almost all the stars in the solar system have an atmosphere, although this varies according to the gases that compose it. The Earth's atmosphere is essential for sustaining life on Earth: it protects it from the Sun's harmful rays and it reduces temperature variation through the greenhouse effect.
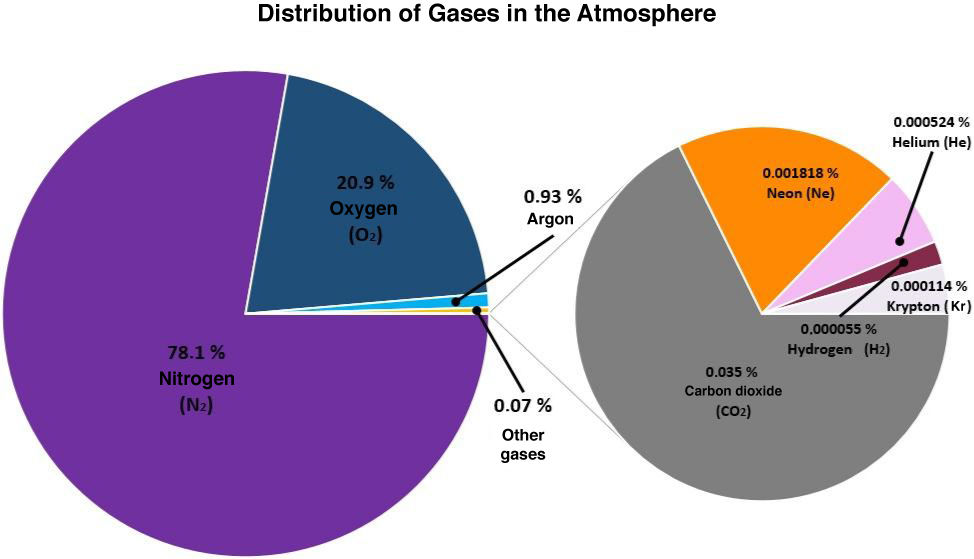
The atmosphere is made up of several gases distributed in different proportions.
-
The most abundant component is nitrogen. This chemical element is essential to life since it is part of the composition of cellular membranes. However, plants and animals cannot absorb nitrogen directly in its gaseous form. Thus, it must be transformed into an assimilable form during the nitrogen cycle.
-
Oxygen is the second most important gas in the Earth's atmosphere. It is essential for life since it is part of the cellular respiration process. Its content in the atmosphere is maintained through the continuous supply of photosynthesis.
-
The remaining portion of the atmosphere is composed of a mixture of several gases including argon, carbon dioxide, helium, methane, and hydrogen.
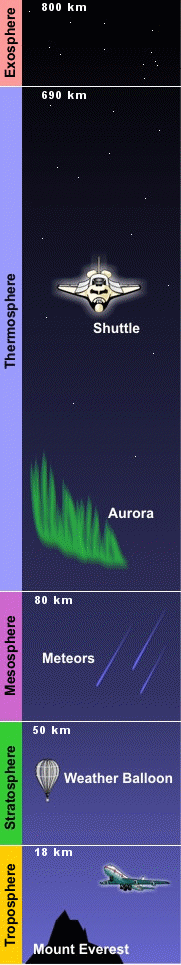
The composition of gases in the Earth's atmosphere varies with altitude. In fact, due to the effect of gravity, the concentration of the air is higher in the lower parts of the atmosphere, and it becomes thinner further up. The exact thickness of the atmosphere is difficult to assess, since at high altitudes the presence of gases is so rare that it is impossible to determine where their presence ends. However, over the years, a lot of information has been collected about the different layers of the atmosphere. Here is a summary.
|
|
Altitude (thickness) |
Layer |
Characteristics |
|
|500| km to ... |
Exosphere |
|
|
|
|85| to |500| km |
Thermosphere |
|
|
|
|50| to |85| km |
Mesosphere |
|
|
|
|12| to |50| km |
Stratosphere |
|
|
|
|0| to |12| km |
Troposphere |
|
Humans make full use of the atmosphere to their advantage, especially the troposphere. They use it for leisure activities, such as paragliding or skydiving.
Humans also use the atmosphere for air transport.
Finally, humans can produce electricity from natural resources in the atmosphere. For example, wind turbines convert the mechanical energy of the wind into electrical energy.
Human activities have largely contributed to the destruction of stratospheric ozone. The layer has thinned by 4 to 6% at mid-latitudes and by nearly 12% at high latitudes (particularly over the Arctic). In fact, releases of chemical compounds containing chlorine and bromine are responsible. In 1890, chlorine-based compounds were discovered. They are called chlorofluorocarbons or CFCs. The following table shows some examples of products in which CFCs are found.
|
Aerosols |
Paints, deodorants, insecticides, shaving cream, whipped cream |
|
Insulating foams |
Foams used in residential and commercial construction |
|
Refrigerants |
Air conditioning (in shops, homes, cars), refrigerators, freezers |
|
Cleaning agents |
Grease removers |
However, since the signing of the Montreal Protocol in 1987, the use of CFCs is no longer authorized, except for uses qualified as critical or essential, or in very minimal and essential quantities, such as in medicine.
The use of fossil fuels is also a source of modification in the composition of the Earth's atmosphere since it increases the quantity of greenhouse gases. For more information on this subject, refer to the concept sheet on climate change.