In general, a model is a visual representation of an abstract situation that is difficult to access or that is hidden. For example, a model can be a list of statements, a drawing or a mock-up.
Thus, the particle model (or corpuscular model) is a scientific model based on the idea that matter is made up of particles. It helps explain certain behaviours and properties of matter.
1. Matter is made up of tiny particles.
All matter is made up of very small particles, more or less spaced apart from each other. These particles can be atoms or molecules. Usually, in the particle model, each atom is represented by a ball. Different colours can be used to distinguish different atoms.
2. A pure substance is made up of identical particles.
In the diagram presented above, it is easy to see that all the water molecules are identical. However, for another pure substance, the molecules would be different from those of water, since they are not composed of the same atoms. The mass and size of the molecules also vary from one pure substance to another.
3. Forces of attraction can hold particles together.
Different types of bonds can be created between the particles of a substance. In general, the closer the particles are to each other, the greater the forces of attraction.
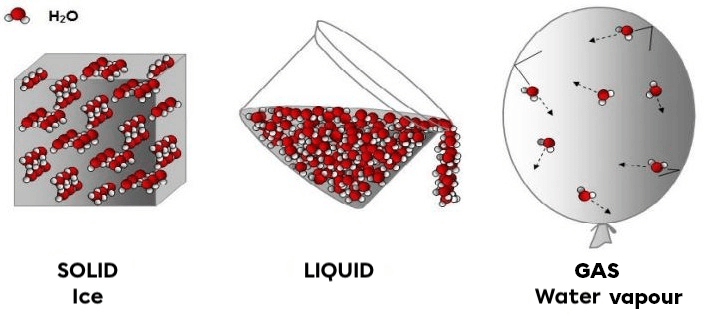
- In a solid state, the particles are very close to one another, therefore very tightly bound, which makes them very ordered.
- In a liquid state, the particles are relatively close to each other, therefore relatively bound, which means that they are poorly ordered.
- In a gaseous state, the particles are very spaced from one another, and, therefore, not very closely bound, which means that they are very disordered.
4. The particles are always in motion.
Each particle has a certain quantity of energy that allows it to move. This motion is subject to the state of the substance. The particles of a solid substance only vibrate, those of a liquid will move slightly relative to each other, while those of a gas move rapidly, and in all directions.
5. If the temperature increases, the speed of particle motion also increases.
The higher the temperature of a substance, the more energy its particles will have. As a result, greater agitation or faster movement of the particles can be observed. However, if the temperature of a substance is low, the opposite situation will be observed, that is less agitation and slower particle motion.
However, this model has limitations:
- It cannot explain the electrical conductivity of a substance.
- It cannot explain chemical transformations.
For more details on the properties of matter according to its state, refer to this concept sheet: The States of Matter.