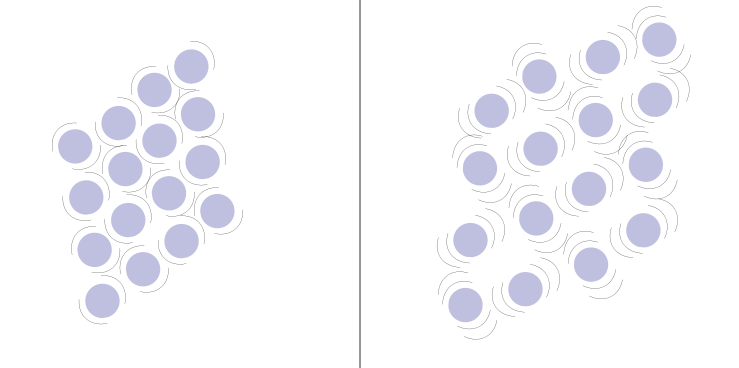
Thermal expansion is the increase in the volume of a substance caused by a rise in its temperature.
In order to understand the concept of thermal expansion, it is important to remember that temperature corresponds to the degree of agitation of the particles that make up a substance. The faster these particles move, the higher the temperature. Consequently, the particles then tend to occupy a larger space due to the acceleration of their motions. This then results in a volume expansion which is sometimes visible to the naked eye. This phenomenon is called thermal expansion of bodies.
In a cold substance, the particles are closer together (left image), but they space out when the temperature rises (right image).
The thermal expansion of bodies affects all substances, regardless of their state, but it is more pronounced for gases than for solids or liquids. For example, if |1\ \text{L}| of air is heated in order to increase its temperature from |0| to |100^\circ\text{C}|, the volume of the air will change to reach approximately |1.36\ \text{L}.| Thus, hot air takes up more space than cold air. Its density is then lower, which is, among other things, at the origin of atmospheric convection currents and helps to explain how a hot air balloon functions.
Air is heated so that the hot air balloon rises in altitude.
If the gas is in a container with a fixed volume (a glass jar for example), the phenomenon of thermal expansion cannot be observed. Rather, there will be an increase in pressure given the intensified motion of particles. This pressure change is possible since gases are compressible fluids. This phenomenon does not therefore involve liquids (incompressible fluids) nor solids.
Solids and liquids also undergo thermal expansion, but on a smaller scale. For example, a metal rod elongates under the effect of a significant increase in temperature. Although almost imperceptible, this thermal expansion must be considered when building large structures, such as bridges or railways, especially in areas where the temperature varies greatly with the seasons. Expansion joints are used between the different bridge beams. Since the materials used to build bridges contract in winter and expand in summer, these expansion joints help to absorb variations in section length and prevent formation of cracks in the materials used to build the bridge.
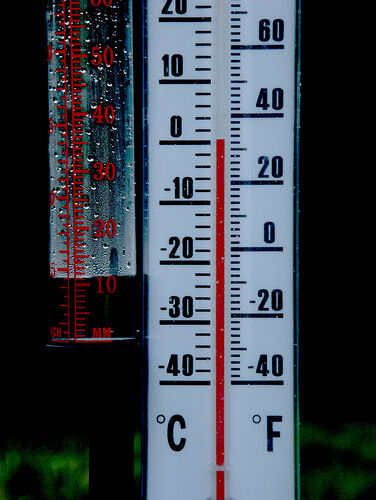
The principle of thermal expansion is used in thermometer operation. A thermometer, made up of a liquid reservoir and a graduated column, makes it possible to measure the temperature, that is, the degree of agitation of the particles. When the temperature is high, the liquid in the thermometer reservoir expands and rises in the graduated column above the reservoir. On the contrary, when the temperature decreases, the liquid contracts and moves down the column. Thermal expansion causes the liquid to contract or expand. Red coloured alcohol or sometimes mercury (although toxic) is usually used in thermometers as both liquids solidify only at very low temperatures.
Temperature can be measured in degrees Celsius or degrees Fahrenheit in certain thermometers.