To predict the effect of a variation in temperature on the state of equilibrium, the energy involved in the reaction must be taken into account. The effect will be different depending on whether the reaction is exothermic or endothermic. According to Le Chatelier's principle, the consequences of a variation in temperature can be summarised as follows:
When the temperature is increased, the reaction at equilibrium tends to oppose this increase in energy.
-
An increase in temperature shifts the equilibrium in favour of the endothermic reaction.
-
A decrease in temperature shifts the equilibrium in favour of the exothermic reaction.
To understand the effect of a variation in temperature on the equilibrium state, let's take the following example:
|N_{2(g)} + 3\; H_{2(g)} \rightleftharpoons 2\; NH_{3(g)} + Energy|
According to Le Chatelier's principle, a system in equilibrium reacts in such a way as to partially oppose the changes imposed on it. So, following an energy input caused by an increase in temperature, the system will react in the opposite way, favouring the direction of the reaction using energy, i.e. the endothermic reaction.
In this example, an increase in temperature creates an imbalance that favours the forward reaction. To return to a new state of equilibrium, the system counteracts by using this excess energy. In this way, the endothermic reaction is favoured, which causes an increase in the reactants in our example; the reverse reaction is then favoured because the energy input forces the product to react.
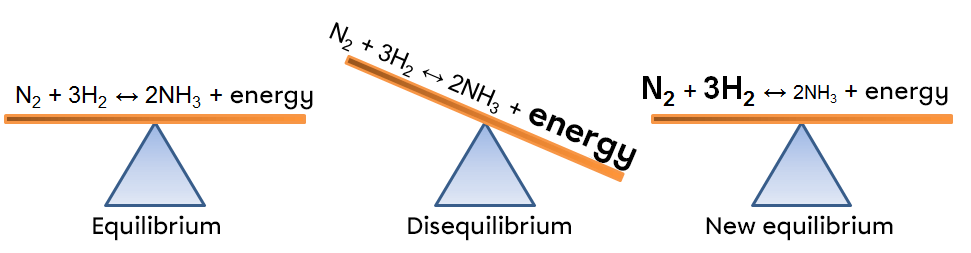
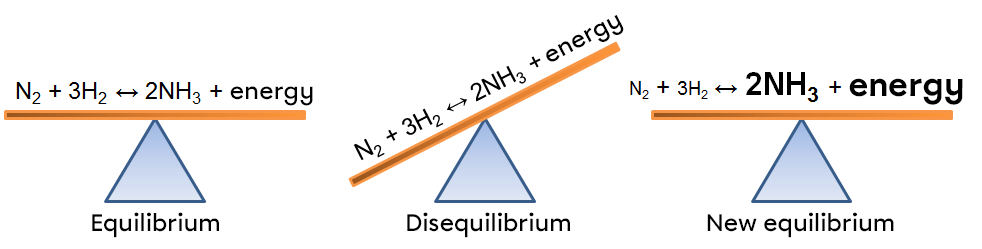
According to Le Chatelier's principle, a system at equilibrium reacts in such a way as to partly oppose the changes imposed on it. Thus, following a withdrawal of energy caused by a decrease in temperature, the system will react in the opposite way, favouring the direction of the reaction that produces energy, i.e. the exothermic reaction.
In this example, a drop in temperature creates an imbalance that favours the opposite reaction. To return to a new state of equilibrium, the system opposes the imbalance by compensating for the lack of energy. In this way, the exothermic reaction is favoured, which causes a decrease in the reactants in our example; the forward reaction is then favoured because the lack of energy forces the reactants to react.
|
Forced change |
Diagram |
Favoured reaction |
|
Increase in temperature |
Reverse reaction
|\large \leftarrow|
|
|
| Forward reaction |\large \rightarrow| |
||
|
Decrease in temperature |
Forward reaction |\large \rightarrow| |
|
| Reverse reaction |\large \leftarrow| |