A change of state, or phase change, is a transformation of matter from one state to another.
The distance between particles in a substance varies according to its state.
-
In solid state, the particles are very close together.
-
In liquid state, the particles are relatively close together.
-
In gaseous state, the particles are far apart.
When the distance between the particles of a substance changes, it can pass from one state to another. The distance between the particles is affected by temperature, among other factors.
- When the temperature of a substance increases, it absorbs energy. The particles become more agitated, move faster and further apart from each other.
- When the temperature of a substance decreases, it releases energy. The particles become less agitated, slow down and move closer to each other.
During a phase change, the substance absorbs or releases energy, but its chemical nature does not change. Therefore, it is a physical change.
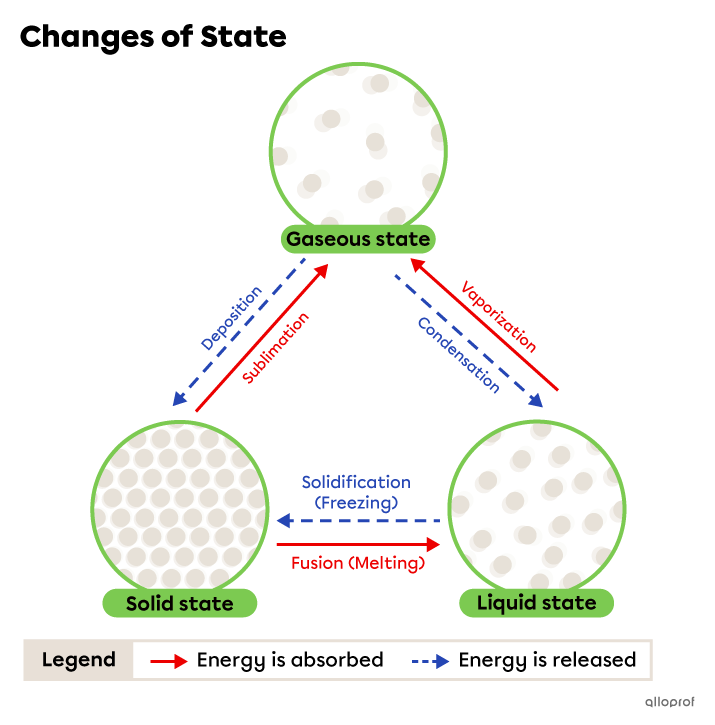
The following phase changes occur when the substance absorbs energy.
The following phase changes occur when the substance releases energy.
It's not just the variation in temperature that allows a substance to go from one state to another. Pressure also influences the state of a substance. High pressure allows particles to stay close together, while low pressure allows them to spread further apart.
For example, propane, which is used to heat homes and barbecues, is in a gaseous state at room temperature and normal pressure. To optimize its transport, it is placed in pressurized cylinders to take up less space. The particles are then compressed together and the propane changes from a gas to a liquid. When the cylinder valve is opened, the propane escapes and its pressure drops. It changes from a liquid to a gas.
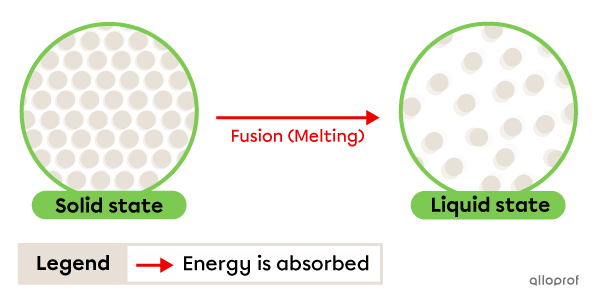
Fusion, often called melting, is the change from solid to liquid state. It occurs due to energy being absorbed by the substance.
The temperature at which this change occurs is called the melting point. This is a physical characteristic property.
Ice melting in the spring is an example of fusion. When the temperature rises, the ice absorbs heat (energy) and becomes liquid.
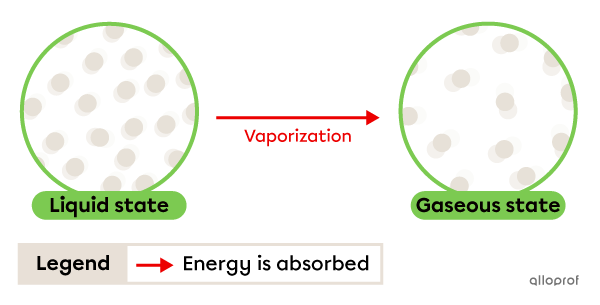
Vaporization is the change from liquid to gaseous state. It occurs due to energy being absorbed by the substance.
When vaporization occurs rapidly due to the input of heat, it is called boiling. The temperature at which this change of state occurs is called the boiling point. This is a physical characteristic property.
When vaporization occurs slowly at room temperature, it is called evaporation. Evaporation takes place at a temperature lower than boiling point.
Boiling water is an example of rapid vaporization. The water absorbs heat (energy) from the stove and becomes gaseous.
Drying wet clothes on a clothesline is also an example of vaporization. To be more precise, it is evaporation: the water evaporates from the fabric into the air and the clothes become dry. It is a slow process that occurs at a temperature that is lower than water’s boiling point.
Although water boils at 100°C, there is still water vapour in the air. It is referred to as humidity. Air can contain a defined amount of water vapour. As long as the humidity in the air does not reach this limit, the water tends to evaporate. Above this threshold, the water no longer evaporates, or barely evaporates. That's why it is easier to dry a towel by a fire, where the air is dry, than in a humid bathroom.
Sublimation is the change from solid to gaseous state. It occurs due to energy being absorbed by the substance.
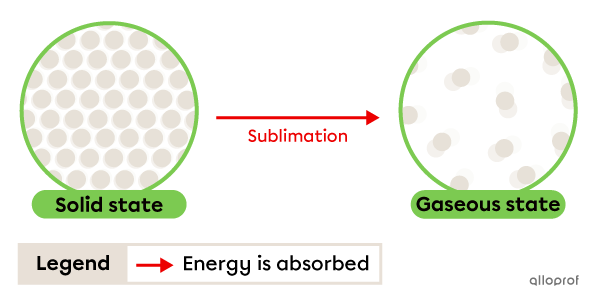
Dry ice is carbon dioxide (CO2) in solid form. Carbon dioxide is solid at temperatures below -78°C and gaseous at temperatures above -78°C. When dry ice is placed in an environment at room temperature, it sublimates. It passes directly from the solid state to the gaseous state, without passing through the liquid state. This is why it is called dry ice.
Solidification, sometimes called freezing, is the change from liquid to solid state. It occurs due to energy being released by the substance.
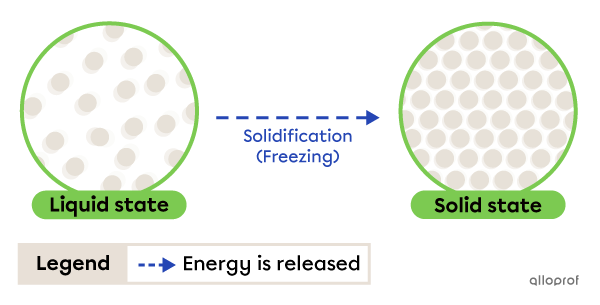
Water freezing on a lake is an example of solidification. As the temperature drops, the water in the lake loses heat (releases energy) and becomes solid.
Condensation is the change from gaseous to liquid state. It occurs due to energy being released by the substance.
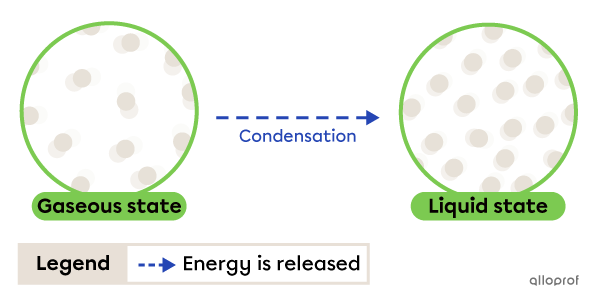
The fog that forms on windows is an example of condensation. When water vapour in the air comes into contact with a cold window, it loses heat (releases energy) and becomes liquid.
Deposition is the change from gaseous to solid state. It occurs due to energy being released by the substance.
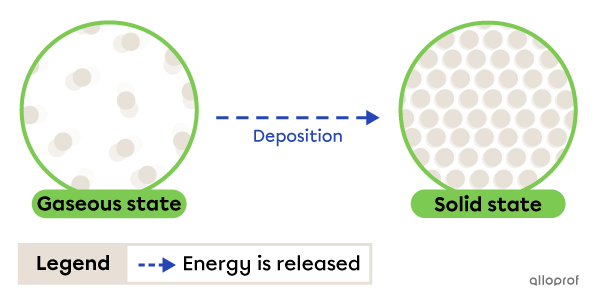
The frost that forms on the lawn in the morning is a result of deposition. When the water vapour in the air comes into contact with cold grass, it loses heat (releases energy) and becomes solid.
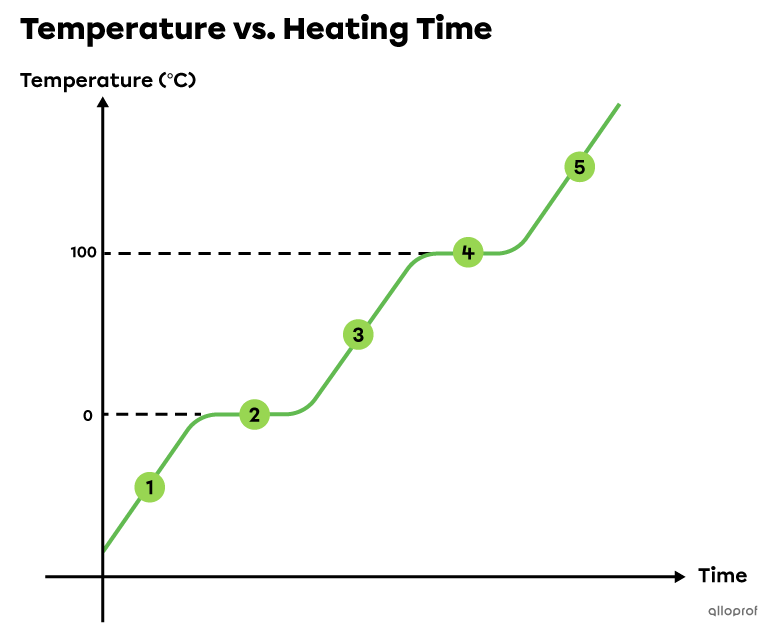
A phase change diagram is a graph showing how the temperature of a substance changes over time. The plateaus correspond to phase changes.
When a substance goes through a phase change, its temperature does not change. All the energy absorbed or released is used to change the substance from one state to another.
If a substance is heated or cooled and its temperature is plotted over time, the horizontal portions of the graph, called plateaus, correspond to phase changes. This diagram can be used to determine the temperatures at which the changes of state occur for a given substance (melting point and boiling point).
When ice is heated in a closed container until it turns completely to vapour, the graph of water temperature over time is similar to this one.
-
All water is solid below 0°C. It absorbs energy (heat) which increases its temperature.
-
At 0°C, water continues absorbing energy but its temperature does not change. The absorbed energy allows solid water to melt and become liquid. Fusion occurs. During this first plateau, water is present in both solid and liquid states. Therefore, 0°C is the melting point of water. At the end of this plateau, all water becomes liquid. Fusion is complete.
-
Between 0°C and 100°C, all water is liquid. It absorbs energy (heat) which increases its temperature.
-
At 100°C, water continues absorbing energy but its temperature does not change. The absorbed energy allows liquid water to boil and become gaseous. Vaporization occurs. During this second plateau, water is present in both liquid and gaseous states. Therefore, 100°C is the boiling point of water. At the end of this plateau, all water becomes gaseous. Vaporization is complete.
-
All water is gaseous above 100°C. It absorbs energy (heat) which increases its temperature.
Alternatively, when water vapour is cooled in a closed container until it turns completely to ice, the graph of water temperature over time is similar to this one.
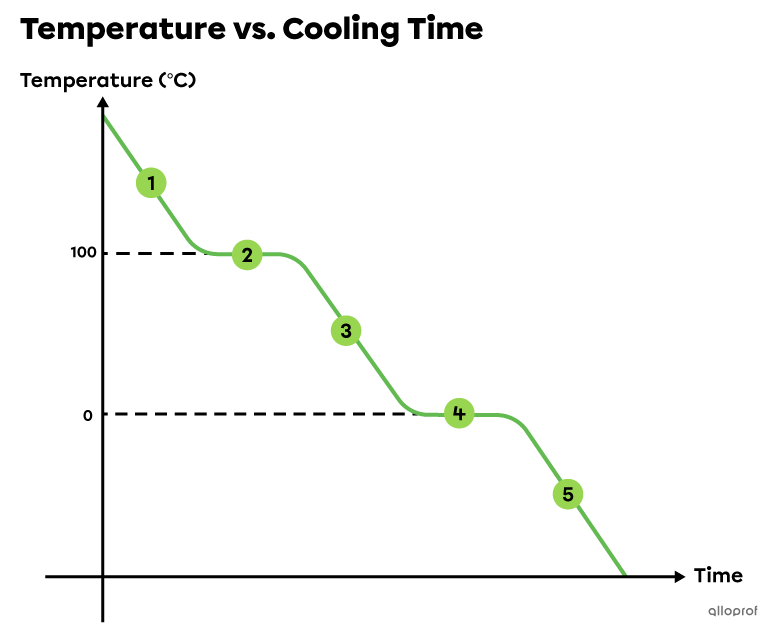
-
All water is gaseous above 100°C. It releases energy (heat) which decreases its temperature.
-
At 100°C, water continues releasing energy but its temperature does not change. The released energy allows gaseous water to become liquid. Condensation occurs. During this first plateau, water is present in both gaseous and liquid states. At the end of this plateau, all water becomes liquid. Condensation is complete.
-
Between 100°C and 0°C, all water is liquid. It releases energy (heat) which decreases its temperature.
-
At 0°C, water continues releasing energy but its temperature does not change. The released energy allows liquid water to freeze and become solid. Solidification occurs. During this second plateau, water is present in both liquid and solid states. At the end of this plateau, all water becomes solid. Solidification is complete.
-
All water is solid below 0°C. It releases energy (heat) which decreases its temperature.
Pour valider ta compréhension à propos des états de la matière de façon interactive, consulte la MiniRécup suivante.








