In the periodic table, the periodicity of properties of the elements refers to the way in which the physical and chemical properties of elements repeat regularly from one period to the next.
Chemical properties are not constant within the same period.
Atomic mass represents the mass of all the particles that make up an atom, which are protons, electrons, and neutrons.
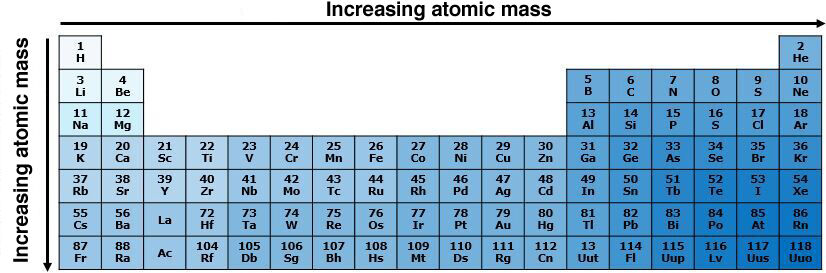
In the same period, the atomic mass increases from left to right in the periodic table. Since the number of particles of the atom increases from left to right with the atomic number, the atomic mass also increases in the same direction — a greater number of particles necessarily implies a greater mass.
In the same group, the atomic mass increases from top to bottom in the periodic table. As the atomic number increases from top to bottom, more protons are found in the atoms at the bottom of the periodic table, which necessarily implies a greater atomic mass.
The atomic radius represents the radius of the atom or, in other words, the radius of the sphere that the atom forms.
The larger the atomic radius, the larger the volume of the atom.
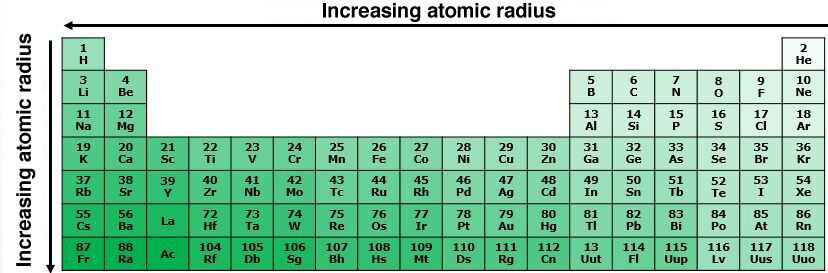
In the same period, the atomic radius increases from right to left in the periodic table. When moving to the right, the atomic number increases, which means that more protons are present in the nucleus. These positive charges exert a greater force of attraction on the electrons located on the electron shells, which brings them closer to the nucleus. The atomic radius is, therefore, smaller for these elements.
Within the same group, the atomic radius increases from top to bottom in the periodic table. While moving down the periodic table, the number of electron shells increases. The electrons, therefore, move further and further away from the nucleus. This contributes to the increase in the atomic radius.
Electronegativity represents the force with which the atomic nucleus attracts electrons from its last electron shell.
The greater the electronegativity, the more difficult it is to remove electrons from the atom, and the easier it is for the atom to accept electrons from surrounding atoms.
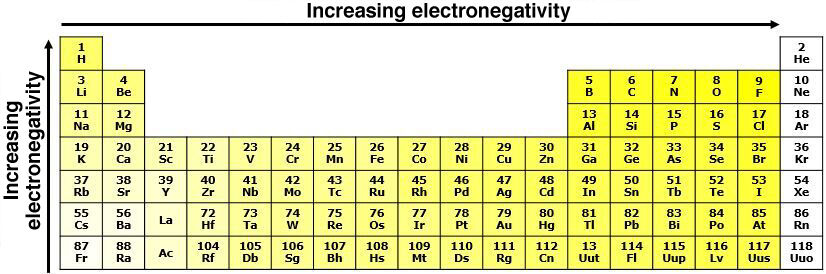
In the same period, electronegativity increases from left to right in the periodic table. As we move to the right, atoms tend to gain electrons in order to acquire a stable electron configuration. Thus, non-metals have a higher tendency to acquire electrons, while this tendency is lower for the elements positioned to the left.
Within the same group, electronegativity increases from the bottom to the top of the periodic table. Since the atoms at the bottom of the periodic table are larger, the force of attraction exerted by the nucleus is weaker given the greater distance between the positive charges of the nucleus and the electrons located on the last electron shell. Therefore, the larger the atom, the lower the electronegativity.
Noble gases have no electronegativity, because these atoms do not seek to attract electrons given their last electron shell is full. Since these atoms already follow the octet rule, they do not seek to attract new electrons.
Ionization energy is the energy required to remove an electron from an atom.
The larger it is, the more difficult it is to strip an electron from that atom.
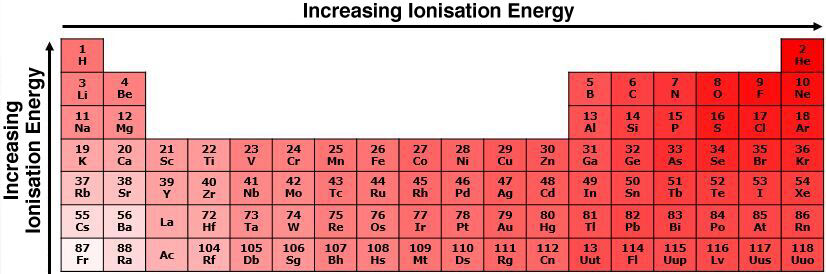
In general, in the same period, the ionization energy increases from left to right in the periodic table. The atomic nuclei of the atoms to the right exert a greater force on the electrons. These electrons therefore require a greater amount of energy to be extracted. In contrast, atoms on the left in the periodic table exert a weaker force on electrons because electrons are further from the nucleus, and fewer protons are present in the nucleus.
In general, within the same group, the ionization energy increases from the bottom to the top of the periodic table. The amount of energy needed to remove an electron is smaller for the bottom elements of the table, because the force of attraction between the valence electrons and the nucleus is smaller. Since these electrons are less attracted, they are easier to pull out than the element located at the top of the table.
Pour valider ta compréhension à propos du tableau périodique de façon interactive, consulte la MiniRécup suivante:
