Choose your level.
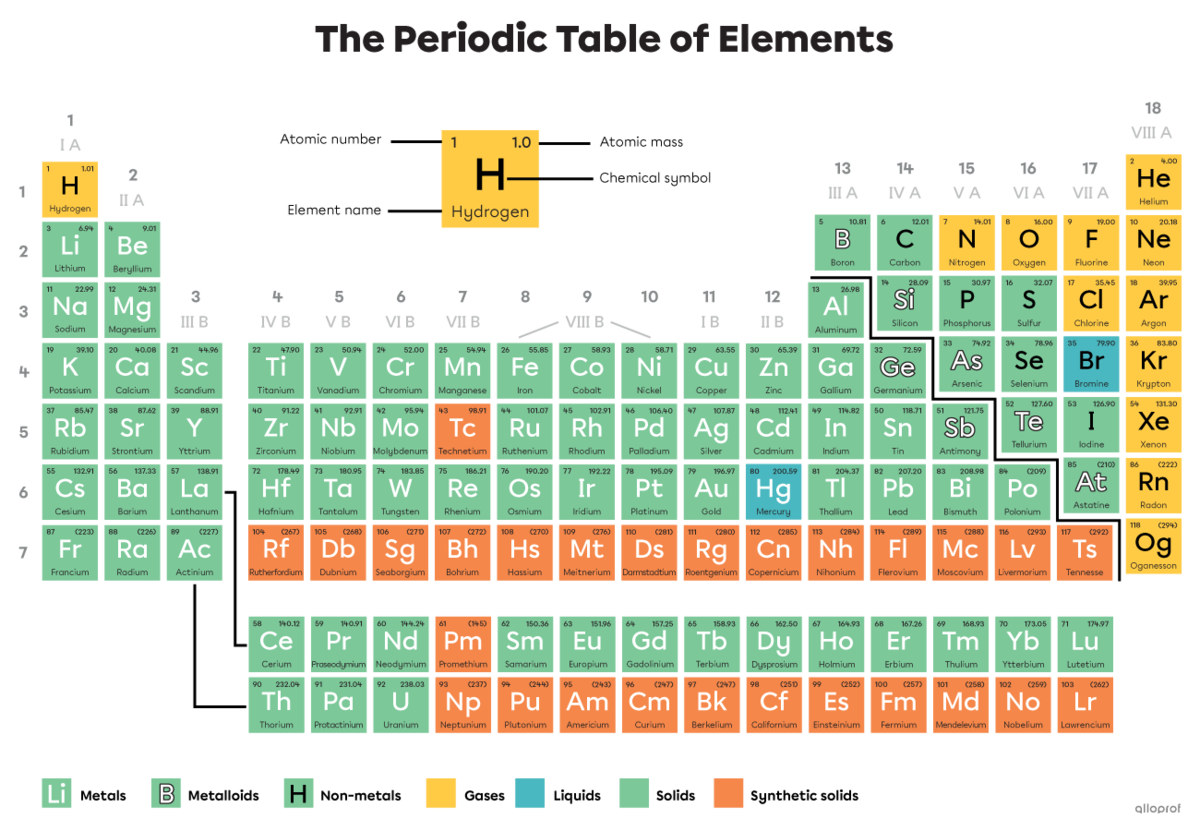
The periodic table groups all chemical elements according to certain characteristics.
During the 1800s, chemists noticed common characteristics between certain elements and wanted to classify them in a logical way. Although a number of chemists considered different classifications, it was the Russian chemist Dmitri Mendeleev who, in 1869, officially presented the structure of the periodic table known today.
This structure enabled him not only to classify the elements discovered at that point, but also to predict the discovery of new elements. Initially, he left empty spaces in the table for elements that were still unknown at the time. Over time, these empty spaces were filled in, confirming his predictions.
Source: Dmitri Ivanovitch Mendeleev, 1897[1].
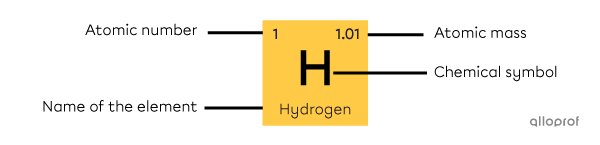
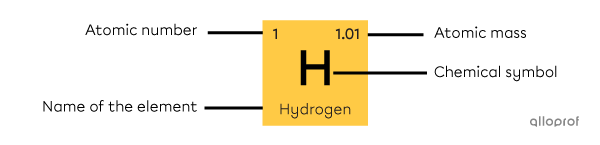
Each square of the periodic table corresponds to a chemical element. These squares contain important information about the element, such as:
-
its atomic number
-
its name
-
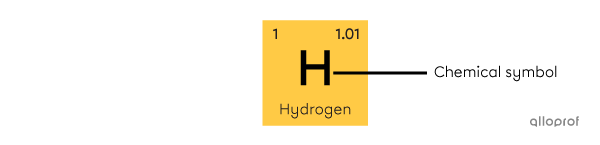
its chemical symbol
-
its atomic mass
The colour of the square also generally gives information about the state of the element.
The information can be located in different parts of the square, depending on the periodic table. It is important to consult the legend of the table to find your way around.
Each element has its own number, known as its atomic number. The 118 elements of the periodic table are classified in ascending order of atomic number, from 1 to 118. The atomic number is assigned to each element according to the internal structure of its atoms.

In addition to having a unique atomic number, each element has a unique name, which is used to identify it in common vocabulary. The origin of these names varies greatly. Some names are given in honour of deities, scientists or places. For example, curium is named after twice Nobel Prize winner Marie Curie. Other names are given according to the properties of the elements. This is the case with iodine, which comes from the Greek word iodes, meaning violet, which is the colour of pure iodine[2]. The name of the element can be different in different languages.

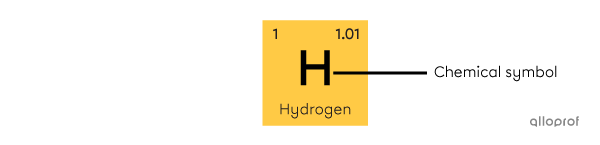
The chemical symbol consists of an uppercase letter, sometimes accompanied by a lowercase letter. The chemical symbol is derived from the Latin name of the element. It is the same in all languages. Often, it also corresponds to the first letters of the name in English, but this is not always the case. Hydrogen (H), helium (He), lithium (Li) and carbon (C) are examples of elements whose symbol corresponds to the first letters of their name. However, the word silver comes from the Old English word siolfur[3]. Silver's chemical symbol is Ag.
The atomic mass of an element is the mass of a single atom of that element. Since the mass of an atom is extremely small, it is generally measured in atomic units (u) to simplify calculations. This is the unit used in the periodic table. One atomic unit corresponds roughly to the mass of hydrogen, the lightest chemical element.

The colour of a square in the periodic table generally indicates the state of the substance at a temperature of |0^\circ\text{C}.| The colours and the state to which they correspond are identified in a legend. In the periodic table at the top of this page, the legend indicates that the yellow squares correspond to elements in gaseous state, blue squares correspond to elements in liquid state, green squares correspond to elements in solid state and orange squares correspond to elements that are synthetic solids. Synthetic solids do not exist in nature; they are produced in laboratories.
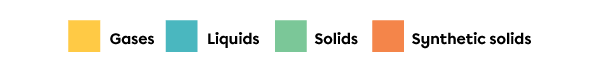
The periodic table organizes all chemical elements according to the composition of their nucleus and their electron configuration.
During the 1800s, chemists noticed common characteristics between certain elements and wanted to classify them in a logical way. Although a number of chemists considered different classifications, it was the Russian chemist Dmitri Mendeleev who, in 1869, officially presented the structure of the periodic table known today.
This structure enabled him not only to classify the elements discovered at that point, but also to predict the discovery of new elements. Initially, he left empty spaces in the table for elements that were still unknown at the time. Over time, these empty spaces were filled in, confirming his predictions.
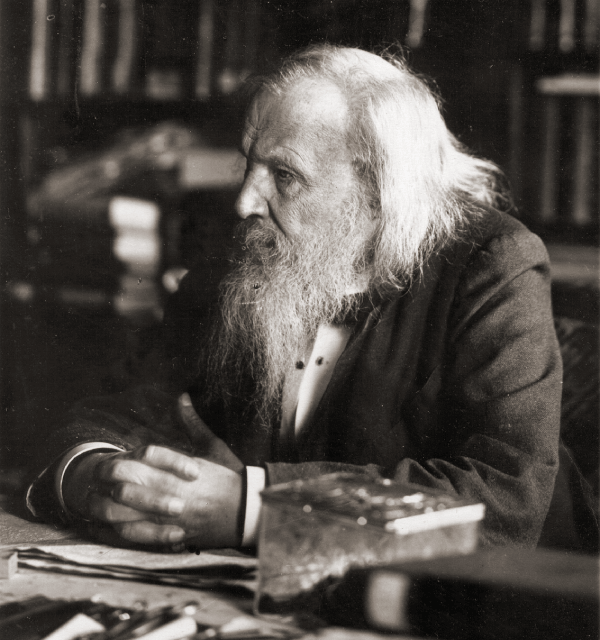
Source: Dmitri Ivanovitch Mendeleev, 1897[1].
Each square of the periodic table corresponds to a chemical element. These squares contain important information about the element, such as:
-
its atomic number
-
its name
-
its chemical symbol
-
its atomic mass
The colour of the square also generally gives information about the state of the element. In some cases, the colour of the chemical symbol indicates whether the element is a metal, a metalloid or a non-metal.
The information can be located in different parts of the square, depending on the periodic table. It is important to consult the legend of the table to find your way around.
Each element has its own number, known as its atomic number. The 118 elements of the periodic table are classified in ascending order of atomic number, from 1 to 118. The atomic number is assigned to each element according to the composition of its nucleus. The atomic number of an element corresponds to the number of protons contained in the nucleus of its atoms.

In addition to having a unique atomic number, each element has a unique name, which is used to identify it in common vocabulary. The origin of these names varies greatly. Some names are given in honour of deities, scientists or places. For example, curium is named after twice Nobel Prize winner Marie Curie. Other names are given according to the properties of the elements. This is the case with iodine, which comes from the Greek word iodes, meaning violet, which is the colour of pure iodine[2]. The name of the element can be different in different languages.

The chemical symbol consists of an uppercase letter, sometimes accompanied by a lowercase letter. The chemical symbol is derived from the Latin name of the element. It is the same in all languages. Often, it also corresponds to the first letters of the name in English, but this is not always the case. Hydrogen |(\text{H}),| helium |(\text{He}),| lithium (|(\text{Li})| and carbon |(\text{C})|are examples of elements whose symbol corresponds to the first letters of their name. However, the word silver comes from the Old English word siolfur[3]. Silver's chemical symbol is |(\text{Ag}).|
The atomic mass of an element is the mass of a single atom of that element. Since the mass of an atom is extremely small, it is generally measured in atomic mass units |(\text{u})| to simplify calculations. This is the unit used in the periodic table. One atomic unit corresponds roughly to the mass of hydrogen, the lightest chemical element.

The colour of a square in the periodic table generally indicates the state of the substance at a temperature of |0^\circ\text{C}| and pressure of |101.3\ \text{kPa}.| The colours and the state to which they correspond are identified in a legend. In the periodic table at the top of this page, the legend indicates that the yellow squares correspond to elements in gaseous state, blue squares correspond to elements in liquid state, green squares correspond to elements in solid state and orange squares correspond to elements that are synthetic solids. Synthetic solids do not exist in nature; they are produced in laboratories.
The colour of the chemical symbol can provide information about the category to which the element belongs, namely whether it is a metal, a metalloid or a non-metal. In the periodic table at the top of this page, the legend indicates that white chemical symbols correspond to metals, white chemical symbols outlined by a black border correspond to metalloids and black chemical symbols correspond to non-metals.

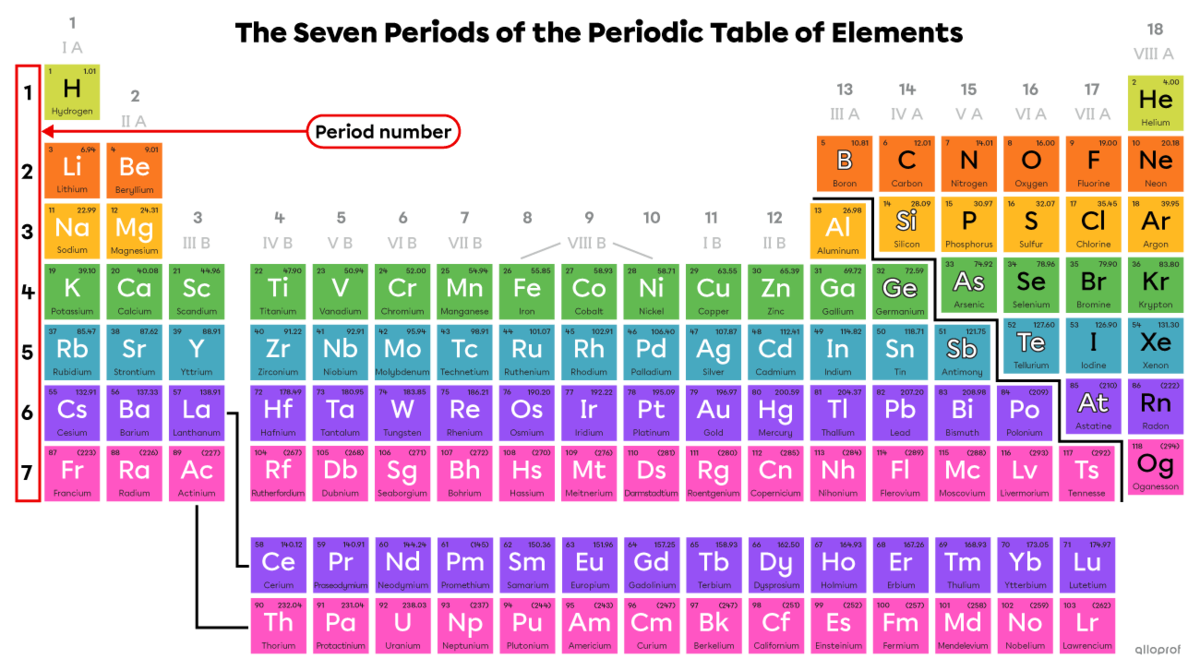
The elements in the periodic table are arranged in rows and columns. The rows correspond to periods and the columns correspond to groups. This classification is based on the electron configuration of the elements. Therefore, it is possible to determine the number of electrons and their arrangement around the atom's nucleus from the position of the element in the periodic table.
The periods indicate the number of electron shells in an atom. There is a maximum of seven. The period number corresponds to the number of electron shells in the atom of an element located in that period.
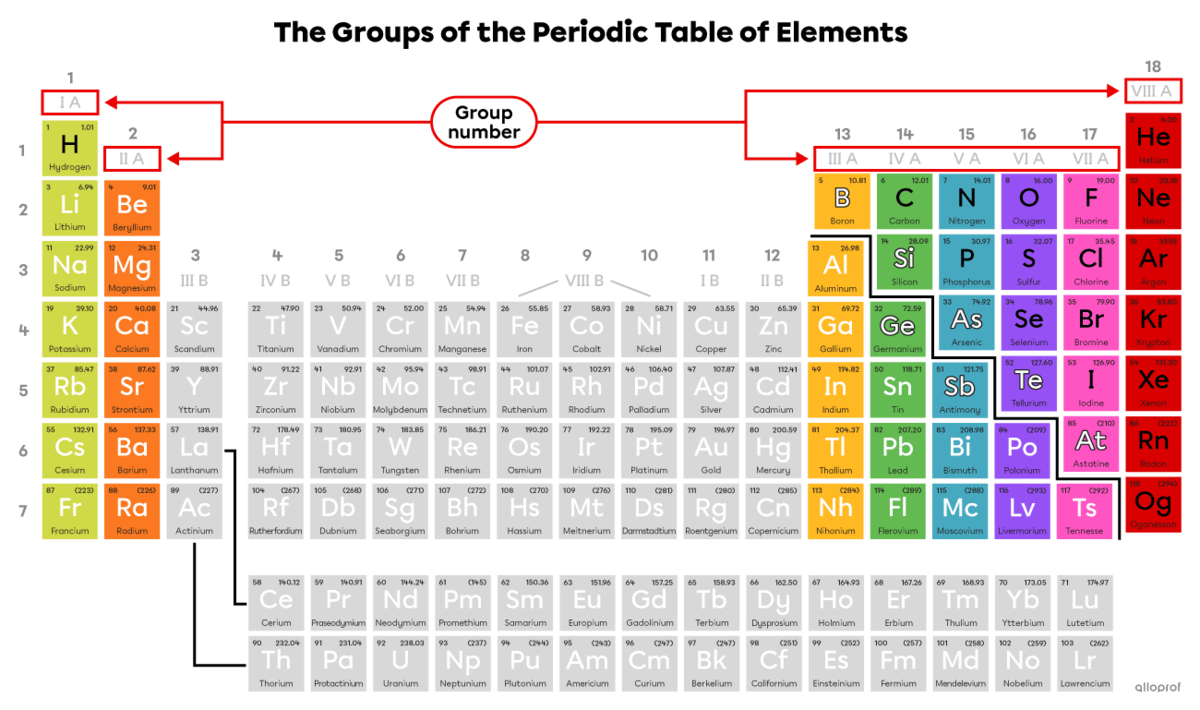
Groups, sometimes called families, indicate the number of electrons on the last electron shell, known as the valence electrons. The group number is identified by Roman numerals. Only groups with an A in their number are discussed in high school. Since they have the same number of valence electrons, elements in the same group have the same chemical reactivity, which is the tendency to react with other elements.
Notes:
-
Hydrogen |(\text{H})| is an exception. It is not part of Group IA. However, a hydrogen atom has 1 valence electron.
-
Helium |(\text{He})| belongs to Group VIIIA, but it has only 2 valence electrons which fill its only electron shell.
The period number and group number are used to illustrate the atom in the following models:
Pour valider ta compréhension à propos du tableau périodique de façon interactive, consulte la MiniRécup suivante :

-
Dmitri Ivanovitch Mendeleev [Photo]. (1897). Wikimedia commons. https://commons.wikimedia.org/wiki/File:DIMendeleevCab.jpg
-
Royal Society of Chemistry (n.d.). Silver. Retrieved May 29, 2023, from https://www.rsc.org/periodic-table/element/47/silver
-
Royal Society of Chemistry (n.d.). Iodine. Retrieved May 29, 2023, from https://www.rsc.org/periodic-table/element/53/iodine