Precipitation is a component of the water cycle. This is the stage during which water, after evaporating, condenses and falls back to earth.
When the water droplets that form clouds become too numerous, the clouds become heavier and emptier. The water then falls in liquid or solid form, depending on the temperature.
Meteorologists use a specific vocabulary to talk about precipitations.
- The nature of the precipitation indicates the state (solid, liquid) of the precipitation.
- The character trait of the precipitation indicates the time (in minutes) that the precipitation lasts (continuous or intermittent downpour).
- The intensity of the precipitation indicates the force and amount that will fall.
Here is a list of the different types of precipitation found in Québec.
|
Rain |
 source |
This is liquid precipitation with drops of 0.5 mm or more in diameter. |
|
Mist |
 source |
This is liquid precipitation with droplets less than 0.5 mm in diameter. |
|
Snow |
 source |
This is solid precipitation formed from ice crystals. |
|
Ice Pellets |
 source |
This is solid precipitation consisting of small balls of ice, often with liquid interiors. |
|
Hail |
 source |
This is solid precipitation in the form of balls composed of several layers of ice (like the peel of an onion). These layers come from successive falls and rises in a storm cloud. This type of precipitation is larger in diameter than ice pellets. It can vary between 5 and 50 mm. This type of precipitation can be observed in summer. |
|
Freezing Rain (or Freezing Mist) |
 source |
This is precipitation that freezes when it hits the cold ground or a very cold object. The resulting layer of ice is called glaze ice. |
Surface, ground and atmospheric waters contain carbon dioxide, a gas that is highly soluble in water.
Carbon dioxide |(CO_2)| produces, in the presence of water |(H_2O)|, ions |H^+| according to the following overall chemical reaction:
|CO_{2} + H_{2}O \rightarrow HCO_{3}^- + H^{+}|
This is why rainwater is slightly acid. Normal rainwater has a pH of around 5.6.
With pollution, the degree of acidity in rain tends to increase, i.e. its pH decreases. It's important to remember that it's not just rain that is acid, but all forms of precipitations, such as snow and hail.
The acidification of rain is caused by the release of sulphur oxides |(SO_x)| and nitrogen oxides |(NO_x)|. These emissions can be produced naturally: by lightning, forest fires, biological decomposition and volcanic eruptions. However, human activity is also responsible for these emissions. Car traffic and numerous industries such as pulp and paper mills, oil refineries and thermal power stations are the biggest producers of these emissions.
Combined with humidity, sulphur oxides |(SO_x)| and nitrogen oxides |(NO_x)| form sulphuric acid and nitric acid, following the following chemical reactions:
Formation of sulphuric acid:
|2 SO_2 + O_2 \rightarrow 2 SO_3|
|SO_3 + H_2O \rightarrow H_2SO_4|
Formation of nitric acid:
|2 NO + O_2 \rightarrow 2 NO_2|
|3 NO_2 + H_2O \rightarrow 2 HNO_3 + NO|
Acid rains have major impacts on fauna and flora. They harm fragile ecosystems that cannot withstand an environment that is too acid. Some places do not have the capacity to neutralise acidity (buffering capacity) because they lack carbonates.
The provinces on the Canadian Shield, such as Ontario, Québec, New Brunswick and Nova Scotia, are the hardest hit by acid rains. Despite efforts to combat atmospheric pollution, it continues to increase, and the rains are becoming more and more acidic.
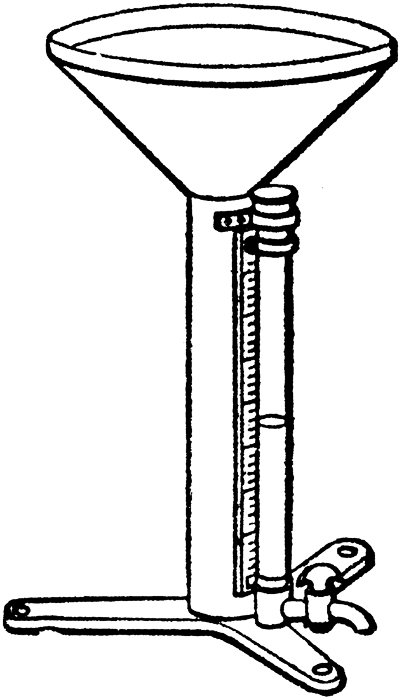
A rain gauge or pluviometer is a meteorological instrument designed to measure the amount of precipitation that has fallen over a given period of time in a given location.
The invention of the rain gauge is attributed to Castelli, in 1639, but the rain gauge is one of the oldest measurement instruments: it was used 400 years BC.
There are two important components to a rain gauge. The upper component receives the precipitation. It is shaped like a funnel to collect the precipitation and reduce evaporation. The precipitation drains towards the lower component of the rain gauge. The lower component is simply a graduated cylinder.
The snow scale is an instrument used to measure the amount of snow on the ground at the time of reading.
A snow scale is a pole driven perpendicularly into the ground. There is a scale on the pole and the zero point is at ground level.
A level gauge is an instrument used to measure the amount of snow that has fallen between two readings.
The best-known level gauge is the Nipher level gauge. It consists of a funnel open to the sky that collects the snowflakes. The snow then falls into a cylinder that can be heated. The snow is transformed into water and the number of millilitres collected can be measured. Knowing that one millilitre of water is equivalent to one centimetre of snowfall, we can deduce the amount of snow that has fallen.