Democritus was an ancient philosopher who lived around 400 BC. He developed the first atomic model.
Democritus asserted that matter is made up of very small particles that cannot be broken or divided. He called these particles atoms (atomos in Greek, which means indivisible). According to Democritus, these particles are separated by a vacuum. This is why his representation of matter is called the model of discontinuity.

Natata, Shutterstock.com
According to Democritus, the way in which atoms are distributed in matter would explain why a substance would have different properties from another substance. For example, the fact that lead is heavier than cork could be explained by the greater amount of atoms in it.
About 100 years later, the philosopher Aristotle opposed Democritus' idea. According to Aristotle, matter must completely fill the space it occupies. There is no vacuum. This is why his idea is known as the model of continuity.

Natata, Shutterstock.com

Aristotle asserted that matter is infinitely divisible, contrary to Democritus, who thought that there are indivisible particles called atoms.
Aristotle also thought that matter is made up of four elements: earth, fire, air and water. According to him, these elements are mixed in different proportions to form the various substances surrounding us.
At that time, people believed more in Aristotle's theory than that of Democritus, even though we know today that Democritus' model is closer to reality.
John Dalton was an English chemist and physicist who lived between 1766 and 1844. Unlike his two predecessors, Democritus and Aristotle, Dalton built his model from scientific experiments based on his own observations and on those of various scientists.

Naci Yavuz, Shutterstock.com
Dalton observed that some gases dissolve better in water than other gases. After analysis, he suggested that gases (made up of atoms) are not all identical. He thought that if some gases dissolve more easily than others, it is because the atoms which make them up have different masses.
Dalton also drew on the work of chemists Joseph Proust and Antoine Laurent de Lavoisier. Proust observed that each substance is always divided into the same products and in the same proportions. Lavoisier demonstrated that during a chemical reaction, the mass of the reactants before the experiment is always equal to the mass of the products after the experiment, and hence his famous sentence: "Nothing is lost, nothing is created, everything is transformed."
Following his observations, Dalton proposed a new atomic model, which he called the atomic theory. It sets out 4 important points.
- Matter is made up of small, invisible and indivisible particles called atoms.
- Atoms of the same element are identical. They have the same properties and the same mass.
- Atoms of different elements have different properties and masses.
- Atoms can combine to form a new substance (a molecule). The produced molecule has different properties from the atoms that constitute it.
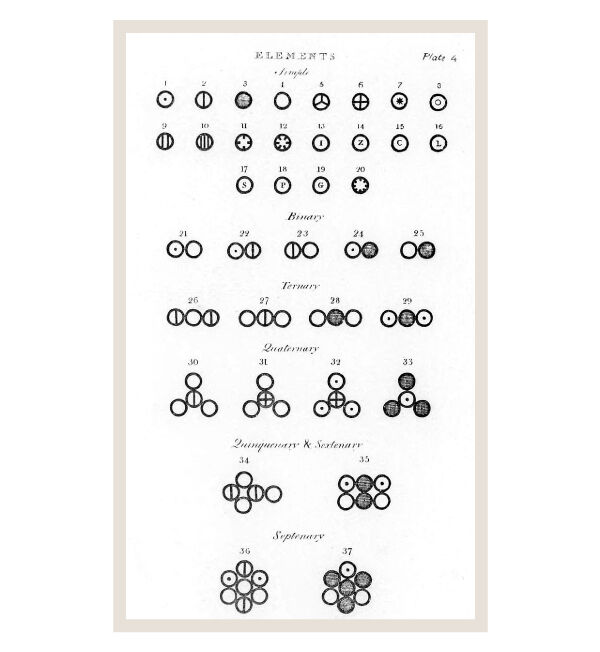
The representation of atoms and molecules by Dalton from his work entitled “A new system of chemical philosophy”, published in 1808.
For more information on this atomic model and to be able to draw the atoms and the molecules according to Dalton’s representation, refer to the concept sheet on Dalton's atomic model.
Joseph John Thomson was a British physicist who lived between 1856 and 1940. He discovered a small particle, the electron, which led him to modify the Dalton model. The study of a ray observed in cathode ray tubes led Thomson to make his discovery.

Natata, Shutterstock.com
In Thomson's time, scientists had already started experimenting with electricity using vacuum tubes. The tubes are called cathode ray tubes, and they have a negative electrode called the cathode, and a positive electrode called the anode. By subjecting these electrodes to an electric current, scientists observed a beam of light, which appeared to come from the cathode, and called it the cathode ray. At that time, scientists did not yet know the nature of the observed ray. Thomson then investigated the matter.
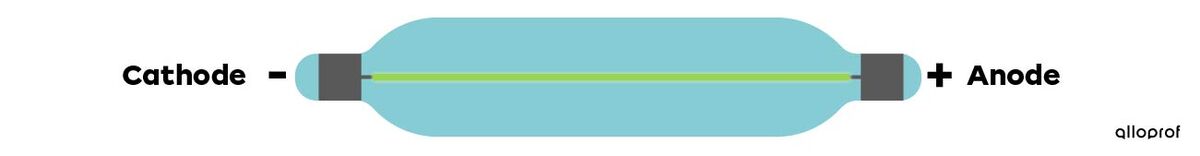
Thomson did a series of experiments and reached two conclusions: the cathode ray is made up of particles smaller than an atom, and these particles are negatively charged. He called this new particle the electron.
In the light of his experiments, Thomson assumed that the atom is not indivisible. In fact, he asserted that it is partly made up of electrons. So in 1904, he proposed a new atomic model.
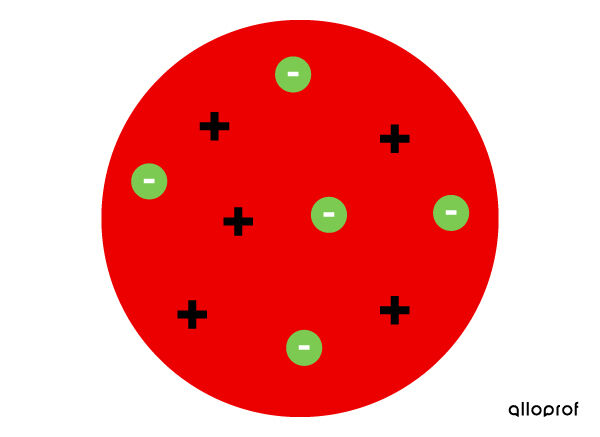
- The atom is not indivisible, since it is possible to detach electrons from it.
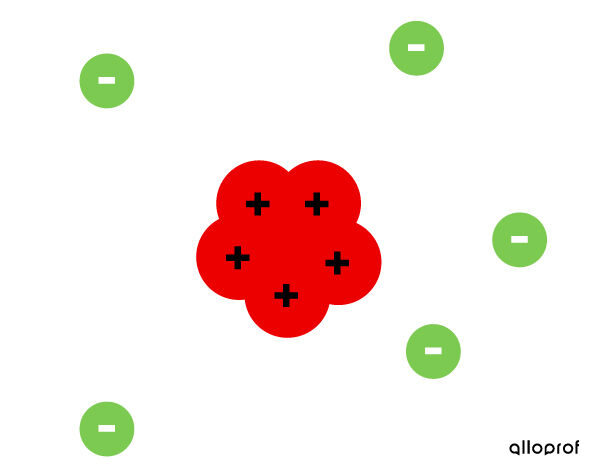
- The atom is a ball of positive matter dotted with small negative grains, the electrons.
- The negative charges of the electrons are counterbalanced by the positive charge of the ball. Thus, the atom is neutral.
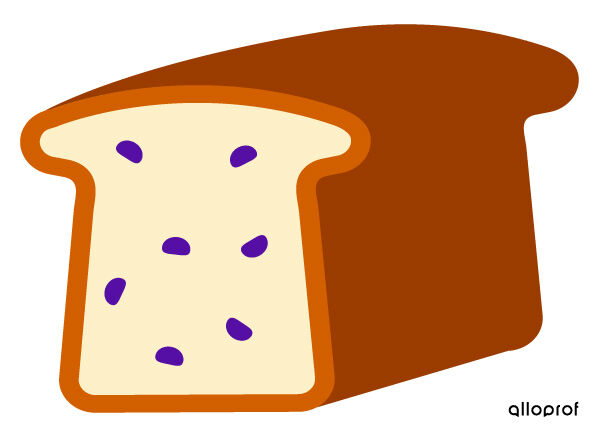
Thomson's atomic model is called the plum pudding model, since we can compare the pudding to the ball of positive matter and the plums to the negative electrons.
Ernest Rutherford was a New Zealand physicist who lived between 1871 and 1937. During an experiment, he made a surprising discovery which led him to modify the previous atomic model: the atom is mostly empty. In addition, he discovered a new subatomic particle, the proton.

Rutherford was interested in radioactivity, specifically in the observations made by scientists of his time who noticed that radioactive elements release different types of radiation. Among these types of radiation is alpha radiation, which is made up of positively charged particles.
From these findings, Rutherford conducted experiments. He bombarded thin sheets of gold foil with these positively charged particles and noticed that the majority of the particles pass through the gold foil, and that a few deviate from their path.
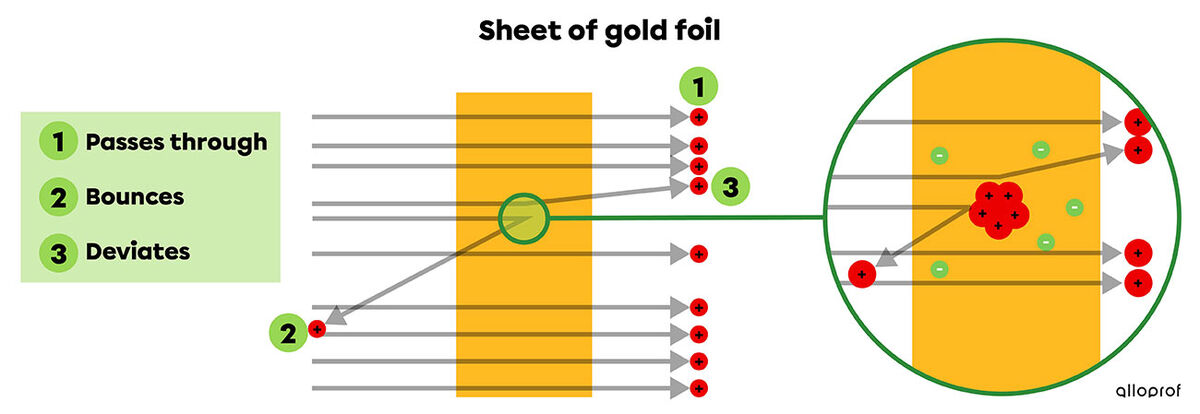
Rutheford concluded that the atom is mostly empty space, since the majority of the alpha particles pass through the sheet (1). He also concluded that the atom has a small, dense nucleus at its center, on which particles either bounce off (2) or deviate from their path (3). This nucleus is made up of positive particles, which he calls protons.
- The atom is mainly made up of empty space.
- The atom has a small, dense nucleus made up of positive particles, called protons.
- Electrons revolve around the nucleus.
- A neutral atom has as many electrons as there are protons.
However, this model has some limitations. Among other things, it does not explain why the electrons of negative charges do not crash into the nucleus of a positive charge.
Niels Bohr was a Danish physicist and student of Rutherford. He brought a precision to the model created by his professor who explained why the negatively-charged electrons do not crash into the positively-charged nucleus. These details also explain the emission lines, which are colours of light emitted when energy is given to a substance.

Naci Yavuz, Shutterstock.com
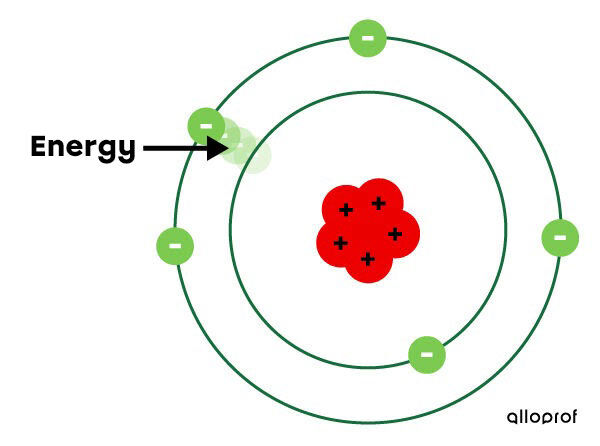
Bohr assumed that electrons travel in orbits, which he called electron shells. Each electron shell corresponds to a specific energy level. The farther away the electron is from the nucleus, the more energy it carries.
Electrons can jump from one layer to another depending on their energy gain or loss. If energy is given to an electron, it moves to a higher shell.
However, the electron does not stay on the higher shell (higher energy level). It returns to its initial electron shell. As it descends, it loses energy, which it diffuses in the form of light.
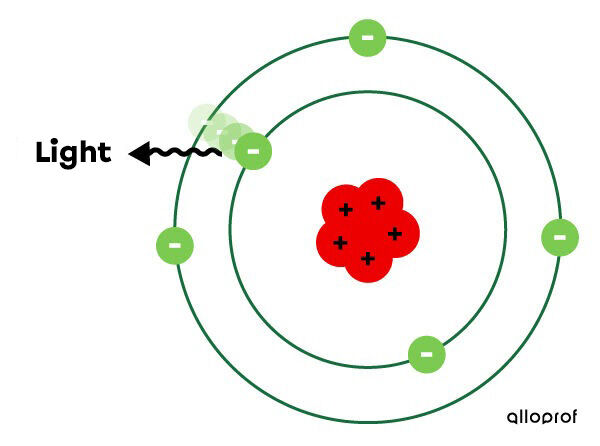
Depending on the movements of an electron (its departure and arrival at various energy levels), the colours of light emitted will be different. In fact, each element of the periodic table has its own spectrum of light lines.
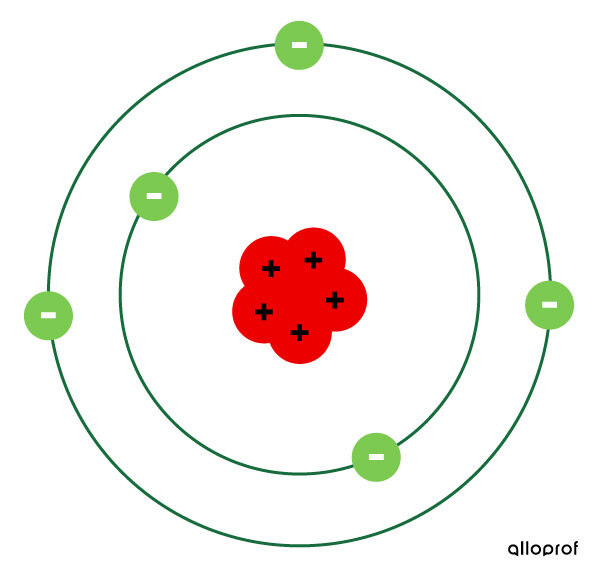
- The atom is represented as mostly empty space with a dense nucleus containing the protons in the center.
- Electrons circulate around the nucleus of the atom in electron shells.
- There can be more than one electron per electron shell.
- A neutral atom has as many electrons as there are protons.
This model is still not perfect. It doesn't explain how the protons, all of which are positively charged, stay bound together in the nucleus instead of repelling each other.
To find out how to illustrate atoms according to the Rutherford-Bohr representation, refer to the Rutherford-Bohr atomic model concept sheet.
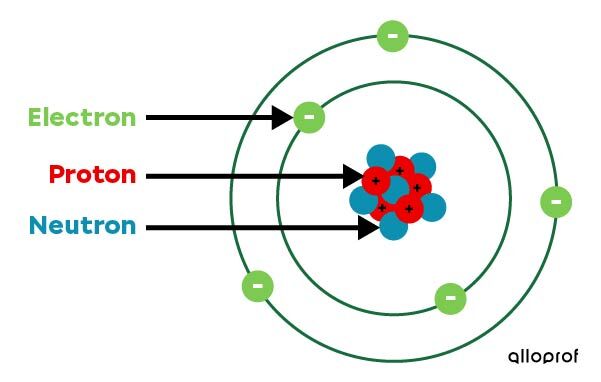
In 1932, the British physicist James Chadwick modified the Rutherford-Bohr model, explaining why the positive particles of the nucleus do not repel each other. He discovered a new particle located in the nucleus. It is called the neutron, as it carries no electric charge.

ROMANVS Roman Mojzis, Shutterstock.com
Neutrons bind to protons in the nucleus. At the same time, they reduce the effect of the proton repulsion, thus allowing the nucleus to be stable.
This model is called the simplified atomic model to differentiate it from the more complex atomic models which were developed in following years.
To illustrate atoms according to the simplified atomic model, refer to the concept sheet on the simplified atomic model.
APS Physics. (2007, mai). May 1932: Chadwick reports the discovery of the neutron.
Bensaada, A., Bolduc, A., Claude, V., Meziane, M., Rhéaume, C. et Tardif, K. (2012). Kaléidoscope ST-STE - 2e cycle (2e année) (2e éd.). Chenelière Éducation.
CEA. (2014, 15 avril). La découverte de l’électron [vidéo].
Couture, I. et Peyronnet, O. (2009). Synergie, 2e cycle (2e année). [Manuel de l'élève]. Chenelière Éducation.
Doud, T. (2014, 08 décembre). The 2,400-year search for the atom [vidéo]. TED-Ed.