The simplified atomic model represents the atom with the number of protons and neutrons in the nucleus, as well as the number of electrons in each of the electron shells.
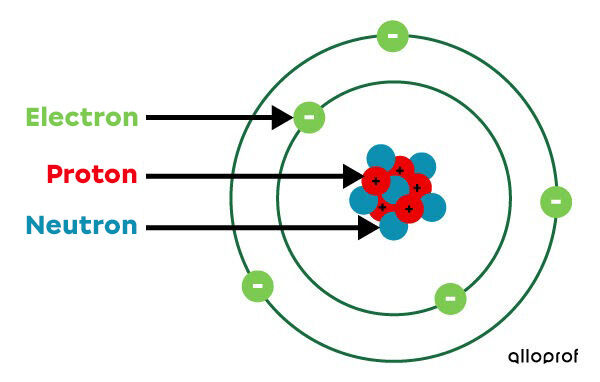
- The atom is represented as mostly empty space with a dense nucleus containing protons and neutrons in the centre.
- The number of neutrons is calculated by subtracting the atomic number from the atomic mass of the element.
- A neutral atom has as many electrons as there are protons.
- Electrons circulate in electron shells around the nucleus of the atom.
To put the simplified atomic model in its historical context and to learn more about the different atomic models, refer to the concept sheet on the history of the atomic model.
To represent an atom according to the simplified atomic model, draw the nucleus indicating its number of protons and neutrons and draw the correct number of electrons on each electron shell by following these 4 steps:
The number of protons in an atom corresponds to its atomic number (Z). This information can be found in the periodic table. To indicate the number of protons, simply draw a circle with the atomic number written in it, accompanied by "p+", which stands for the word proton.
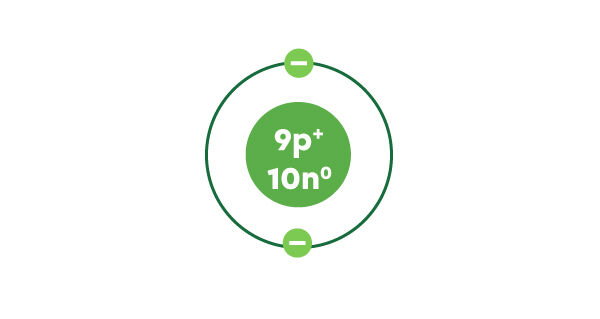
The atomic number of fluorine is 9. It, therefore, has 9 protons.

The atomic mass is the number of protons and neutrons, which are the most massive particles in an atom. To find the number of neutrons, simply round the atomic mass to a whole number, which is equivalent to the mass number, and then subtract the atomic number, which corresponds to the number of protons. Finally, write the number of neutrons in the circle, with the symbol "n0", which stands for neutron.
|\text{N = A - Z}|
where
|\text{N}| represents the number of neutrons
|\text{A}| represents the mass number
|\text{Z}| represents the atomic number, or the number of protons
The atomic mass of fluorine is 18.998. Rounded to a whole number, it gives a mass number of 19.
The atomic number of fluorine is 9.
|\text{N = A - Z}|
|\text{N} =19-9|
|\text{N} =10|
There are 10 neutrons in the nucleus of a fluorine atom.

The number of electrons in a neutral atom is equal to the number of protons. It is, therefore, the same as the atomic number.
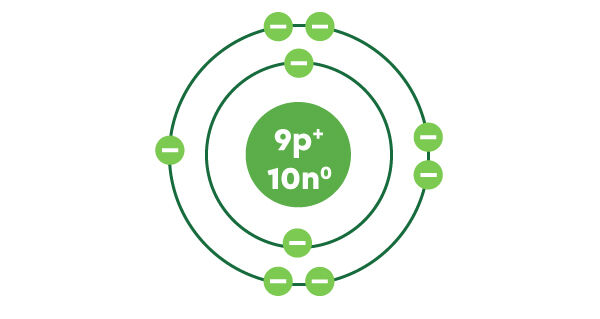
Fluorine has 9 protons. Since it is neutral, it also has 9 electrons.
When distributing electrons on electron shells, the same rules must be observed as for the Rutherford-Bohr atomic model, i.e. the following 3 rules:
- The first shell can contain a maximum of 2 electrons.
- The following shells can each contain a maximum of 8 electrons. This is valid for elements with atomic numbers less than or equal to 20.
- All shells must be filled to a maximum, except the last one, which may be left incomplete.
Electrons are distributed by first filling the electron shell closest to the nucleus, then by continuing with the 2nd, 3rd and 4th shells, if necessary. It is important to respect the maximum number allowed for each electron shell.
Fluorine has 9 electrons in total, and the first electron shell can hold a maximum of 2 electrons. We, therefore, draw 2 electrons in this first shell and now 7 remain to be placed.
The second layer can contain a maximum of 8 electrons, which is where the remaining 7 electrons can be placed. The 9 electrons of fluorine are now illustrated.
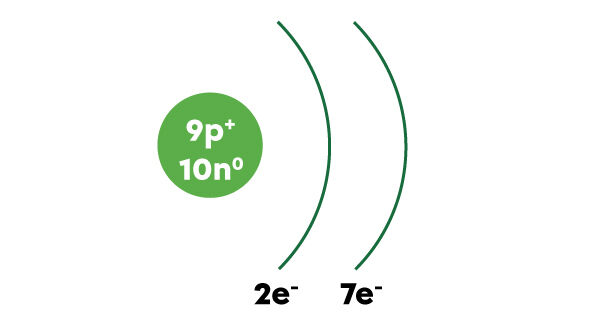
When atoms have many electrons, the drawing may become too complicated. It is possible to draw atoms according to the simplified atomic model in a short form.
Simply draw the nucleus with the number of protons and the number of neutrons indicated. Then draw the arcs of a circle to the right of the nucleus to represent the electron shells. We write the number of electrons in each electron shell under the corresponding layer. We then add the symbol "e-" for electron.
Here is the short form for illustrating the simplified atomic model of fluorine.
Bensaada, A., Bolduc, A., Claude, V., Meziane, M., Rhéaume, C. et Tardif, K. (2012). Kaléidoscope ST-STE - 2e cycle (2e année) (2e éd.). Chenelière Éducation.
APS Physics. (2007, mai) May 1932: Chadwick reports the discovery of the neutron.
Couture, I. et Peyronnet, O. (2009). Synergie, 2e cycle (2e année). [Manuel de l'élève]. Chenelière Éducation.