Acid-base neutralization is a reaction between an acid and a base in which salt and water are formed.
The general equation for acid-base neutralization is as follows:
|\color{red} {\text {Acid}} + \color{blue} {\text {Base}} \rightarrow {\text {Salt}} + {\text {Water}}|
This reaction can also be written in the following form:
|\color{red} {\text {H}}{\text {A}} + {\text {B}}\color{blue} {\text {OH}} \rightarrow {\text {BA}} + \color{red} {\text {H}}\color{blue} {\text {OH}}|
Here are some examples of acid-base neutralization reactions.
|\color{red} {HCl} + \color{blue} {NaOH} \rightarrow {NaCl} + {H_{2}O}|
|\color{red} {H_{2}SO_{4}} + \color{blue} {Ba(OH)_{2}} \rightarrow {BaSO_{4}} + 2 {H_{2}O}|
Alternatively, a neutralization can be represented according to the particle model.
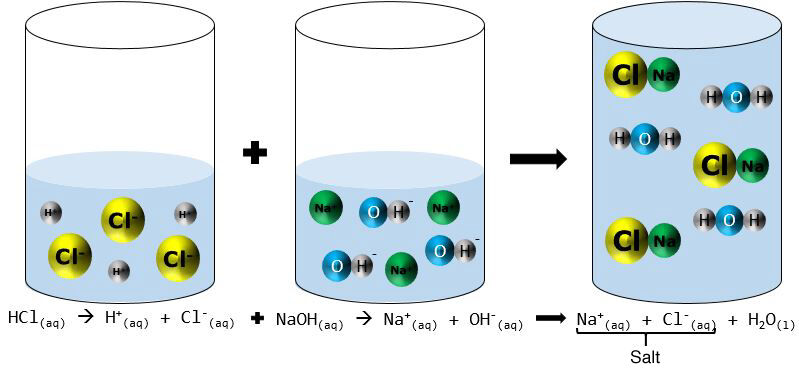
In an aqueous medium, hydrochloric acid |(HCl)| separates to form two ions, |H^{+}| and |Cl^{-}| . The base, sodium hydroxide |(NaOH),| also dissociates into ions, |Na^{+}| and |OH^{-}.| When the acid is mixed with the base, the ions |H^{+}| and |OH^{-}| react together to form water. The other two ions, |Na^{+}| and |Cl^{-},| combine to form a salt called sodium chloride |(NaCl).|
In summary, when we neutralize a substance, we want to bring its pH as close as possible to 7. The |H^{+}| ions and the |OH^{-}| ions must be in the same quantity for the solution to be neutral.
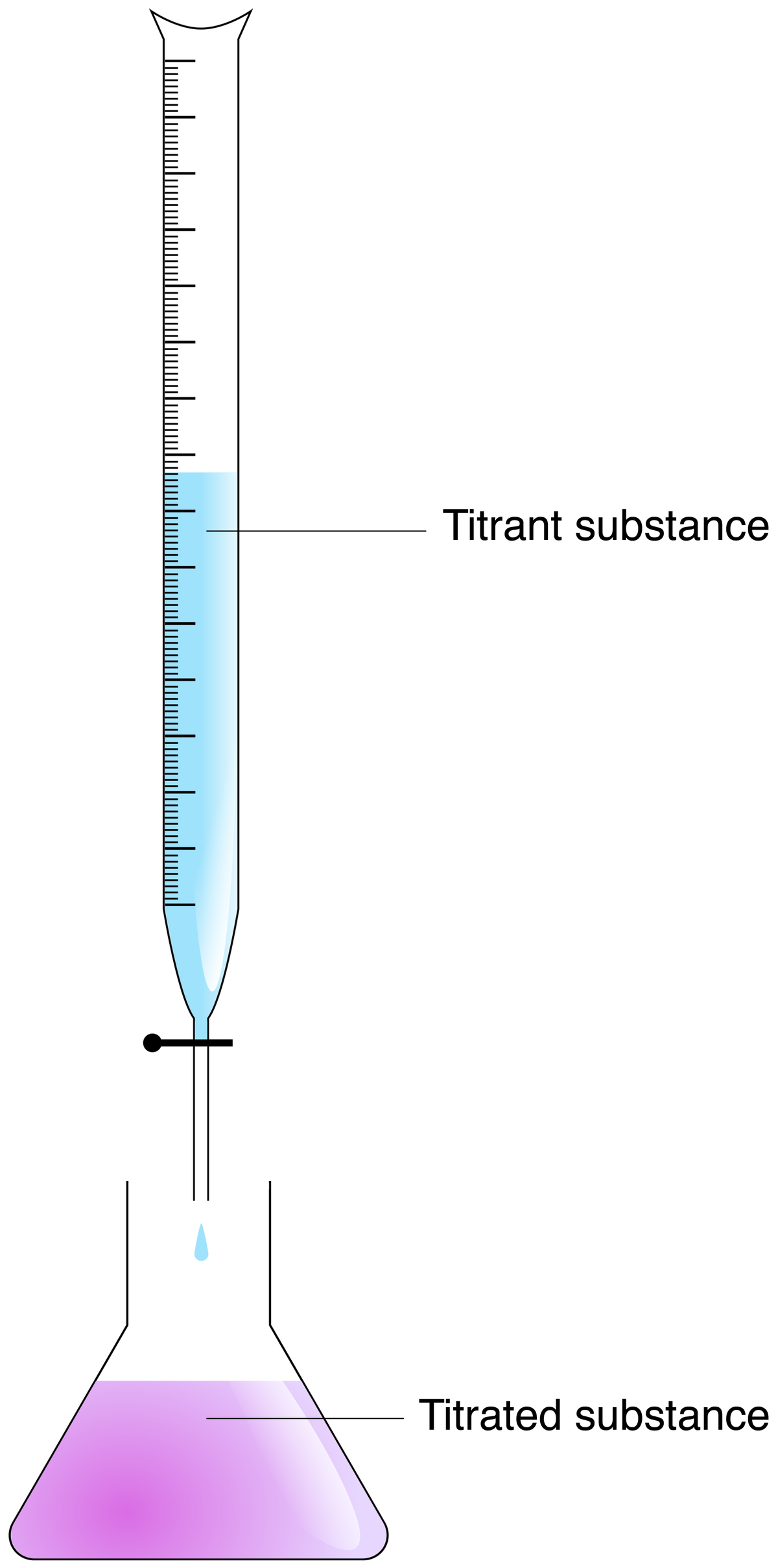
In the laboratory, acid-base neutralization is usually done using a burette and an acid-base indicator. Add a few drops of acid-base indicator to the solution to be neutralized. Depending on the colour obtained with the indicator, it will be necessary to neutralize with an acid (if the solution is basic) or with a base (if the solution is acidic). A neutralizing solution must then be added drop by drop until the solution is neutralized, that is until there are as many |H{+}| ions as |OH^{-}| ions.
An acid can be neutralized with a carbonate as in the following example.
|2 HCl + CaCO_{3} \rightarrow CaCl_{2} + H_{2}O + CO_{2}|
In this neutralization reaction, carbon dioxide |(CO_{2})| will also be produced. We can therefore observe effervescence during this reaction.
Certain acid-base neutralization reactions are present in everyday life. For example, to neutralize the acidity of a lake or soil, lime must be added.
Acidity in the digestive system can also be neutralized by using antacids.