This concept sheet explains the procedures to follow to neutralize a substance (acid or base).
Acid-base neutralization is the reaction between an acid and a base. This reaction produces two substances — salt and water. In addition, it is possible to identify the concentration of the acid or base using the experimental data. Titration, on the other hand, enables us to determine the concentration of a solute in a solution based on another solution whose concentration is already known. Neutralization is an example of acid-base titration.
The technique used for neutralization and that for titration are similar. Only the substances used change depending on the type of reaction performed. Both techniques require an indicator so that a colour change can be observed indicating that the reaction is complete.
This concept sheet will explain the acid-base titration.
-
Graduated cylinder
-
Burette
-
Burette stand
-
Universal support
-
Erlenmeyer flask
-
Solution to neutralize
-
Neutralizing solution
-
Acid-base indicator
-
Beaker
-
Apron or smock
-
Safety glasses

Since the intended pH for neutralization is |7,| the preferred indicator for this lab will be bromothymol blue, since the endpoint is between |6.0| and |7.6.| However, the choice of indicator is left to the person doing the lab depending on the purpose of the experiment. The following concept sheet provides examples of indicators that can be used: The pH Scale and Acid-Base Indicators.
-
Measure a certain volume of the solution to be neutralized using a graduated cylinder.

-
Pour the liquid measured in step 1 into an Erlenmeyer flask.
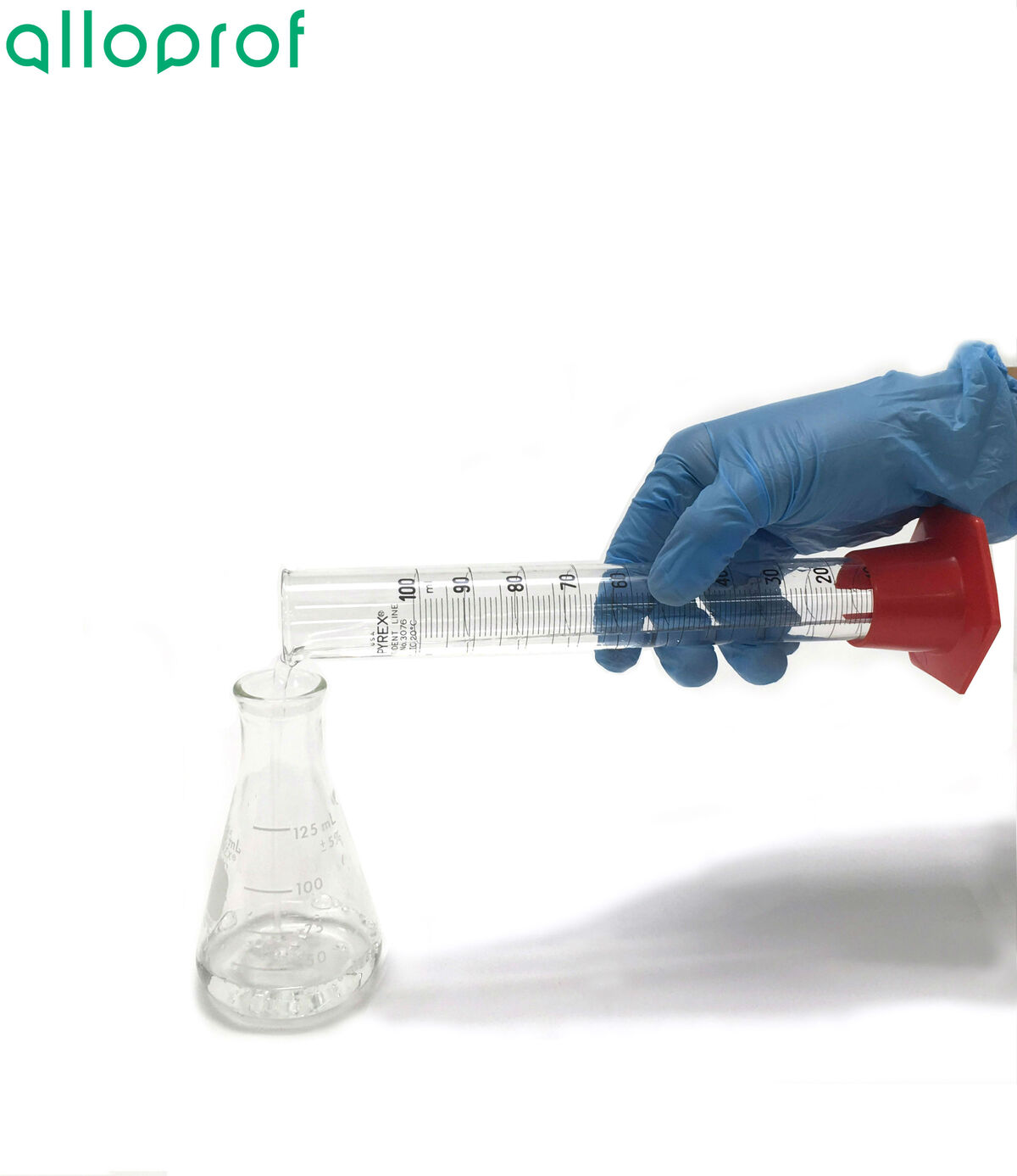
-
Add a few drops of the indicator into the Erlenmeyer flask.

The colour of the solution can indicate the nature of the solution obtained. For example, for bromothymol blue, a yellow colouration indicates that the solution is acidic: it must therefore be neutralized with a base. A blue colouration in the Erlenmeyer flask indicates that the solution is basic and that the neutralizing solution must be acidic.


The amount of indicator to add depends on the titration performed. Generally, when using |10| mL of the solution to be neutralized, four to five drops of indicator are required to adequately observe the colour change indicating that the reaction is complete.
-
Install the burette support on the universal support stand, and the burette in the burette support.

-
Fill the burette to the brim with the neutralizing solution.
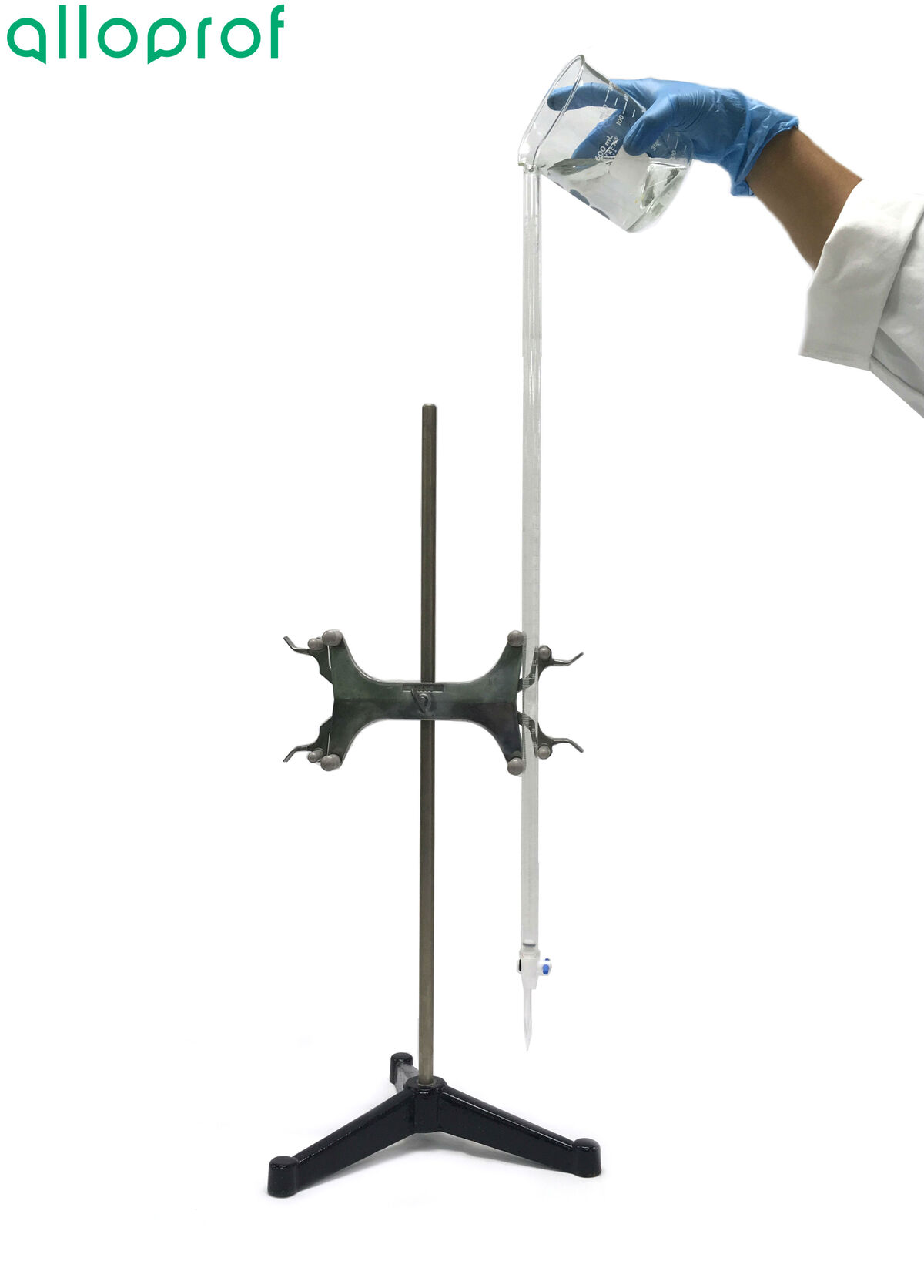
-
Place a beaker under the burette stopcock.

-
Open the burette tap in order to fully fill the part under the stopcock and to adjust the volume of the burette to zero.

-
Place the Erlenmeyer flask under the burette.

-
Slowly open the burette stopcock, and allow the neutralizing solution to flow slowly into the solution to be neutralized in the Erlenmeyer flask while gently swirling the flask.

-
When the solution in the Erlenmeyer flask persistently changes colour at the point where the neutralizing solution comes into contact with the solution to be neutralized, close the tap as to slow down the flow of the neutralizing solution.

-
Add the neutralizing solution drop by drop, stirring continuously, until the colouration is constant.

The desired colouration depends on the indicator used. In the case of bromothymol blue, the colour to be obtained is between the yellow of the acid solution and the blue of the basic solution: the solution must therefore be green to achieve complete neutralization.
-
Record the volume of the neutralizing solution used.

-
Calculate the concentration of the solution to be neutralized.
-
Clean and store equipment.
If possible, it is recommended to repeat the experiment a second time in order to validate the experimental results obtained the first time.
To find the concentration of the solution to be neutralized, experimental data must be used.
What is the concentration of a sample of |50.0 \: \text {mL}| of a solution to be neutralized if |29.5 \: \text {mL}| of a neutralizing solution whose concentration is |0.0150 \: \text {mol/L}?|
The first step is to identify the variables in this situation.
||\begin{align}C_{1} &= x & &\quad & C_{2} &= 0.0150\: \text{mol/L}\\
V_{1} \: &= \: 50.0 \: \text{mL} &&& V_{2} &= \: 29.5 \: \text{mL} \end{align}||
Since there is only one unknown variable, this variable can be determined mathematically.
||\begin{align} C_1\times V_1=C_2\times V_2 \quad \Rightarrow \quad C_1 &=\dfrac{C_2 \times V_2}{V_1}
\\ \\
&= \dfrac{0.0150\: \text{mol/L} \times 29.5 \:\text{mL}}{50.0\: \text{mL}}\\ \\
&= 0.00885 \:\text{mol/L}\end{align}||
The concentration of the solution to be neutralized is therefore |0.00885 \:\text{mol/L}.|
Presenting the experimental values in a table is essential. Here is a sample results table for acid-base titration.
Acid-base titration of a solution
| |
Solution to neutralize |
|
Neutralizing solution |
|
|C_1| |
|0.00885 \: \text {mol/L}| |
|C_2| |
|0.0150 \: \text {mol/L}| |
|
|V_1| |
|50.0 \: \text { mL}| |
|V_2| |
|29.5 \: \text {mL}| |
In some contexts, such as in stoichiometry, calculating the number of moles of the solution to be neutralized may be necessary. In that case, the molar concentration formula should be used to determine the number of moles. It might also be possible to determine the pH of the initial solutions using dissociation equations.