Salinity is the measure of the amount of salts dissolved in a given volume of water.
More than two-thirds of the Earth’s surface is covered with water, whether the water is in the form of seas, oceans, lakes, or glaciers and ice floes. When water is in a liquid state, it contains many dissolved mineral salts. These salts come mainly from the erosion of rocks in the lithosphere from runoff and groundwater. Throughout their journey, the salts are carried away by runoff and accumulate during their progression in the watersheds.

Thais29, Shutterstock.com
Depending on the amount of dissolved salts, there are 3 different types of water.
|
Type of water |
Description |
|---|---|
|
Water is said to be fresh when its salinity is less than |1| g/L. Freshwater is mainly found on continents. It is estimated that only |2.5\ \%| of the water found on Earth is fresh and therefore likely to be used for drinking. |
|
Brackish water forms the transition between freshwater and saltwater areas. The salinity is between |1| and |10| g/L. Brackish water is found mainly at the mouths of rivers, in estuaries, and in deltas. |
|
The water of seas and oceans has a higher salinity than that of fresh water. All waters with a salinity exceeding |10| g/L are classified as salty. Among the many types of salt found in seawater, sodium chloride |(NaCl)| is the most abundant. |
Salinity varies with the depth of the water and from place to place in the oceans. In 2009, the World Ocean Atlas estimated that the average salinity of the oceans was |35| g/L.
The variation in salinity all over the world is partly responsible for ocean circulation. In fact, the amount of salts dissolved in water has an impact on the density of the water. For example, water with a high salinity will have a greater density than water with a lower salinity. Saltier water will, therefore, tend to sink towards the bottom, thus creating a current.
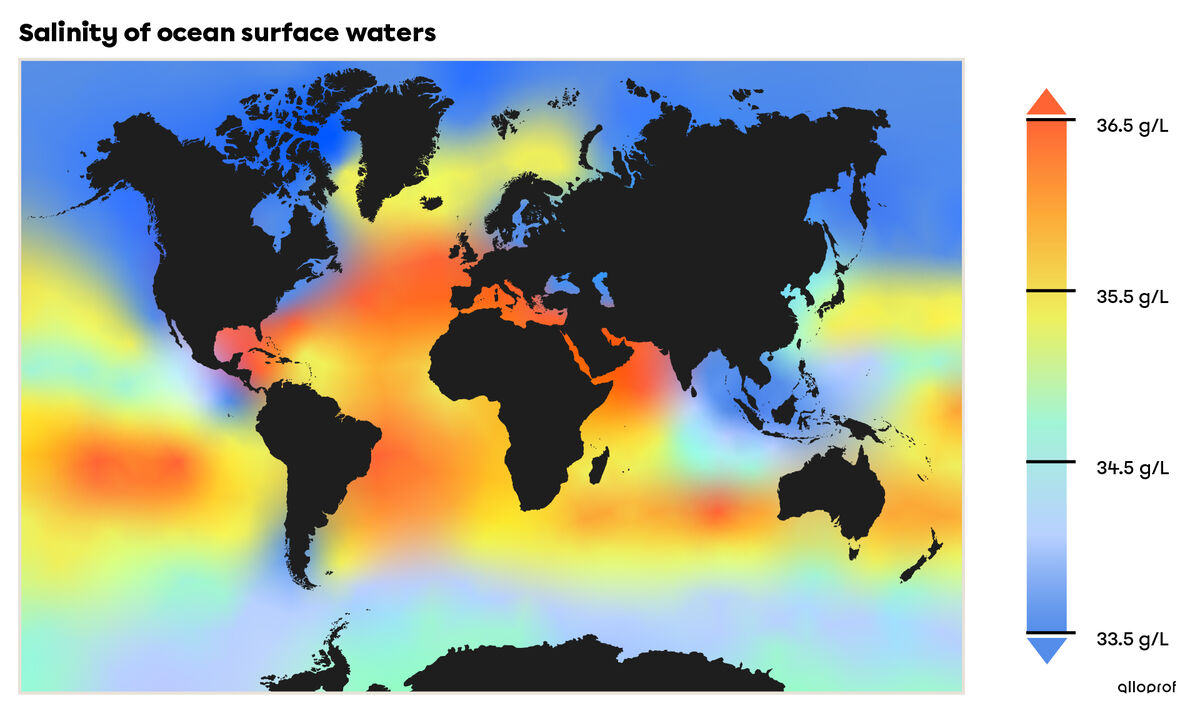
Reference: World Ocean Atlas, 2009
Dissolved salt changes the usual properties of water.
-
Salt water has a lower capacity for dissolving oxygen |(O_2)| than fresh water.
-
Salt water is denser than fresh water, so its density is greater than that of fresh water.
-
The melting point of salt water is lower than that of fresh water, (|-1.9\ ^{\circ}\text{C}| instead of |0\ ^{\circ}\text{C}|), while its boiling point is higher, (|101.5\ ^{\circ}\text{C}| instead of |100\ ^{\circ}\text{C}|).
-
Salt water conducts electricity.
Another phenomenon caused by salinity is the increase in the density of the water. In fact, the density of distilled water is |1\ \text {g/mL}|. However, when salt is added to distilled water, the mass of the solution increases, because there are more particles present in the sample.
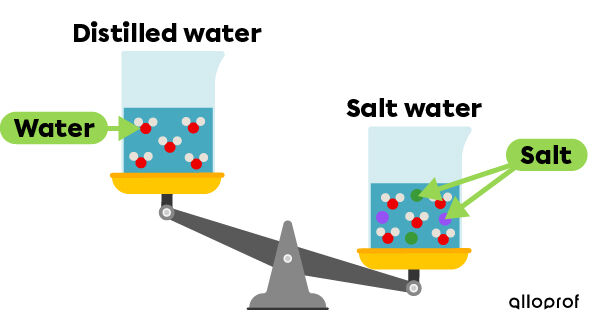
Due to the salts dissolved in the oceans, sea water has a higher density than fresh water. That is why a person is easily able to float on the water in the Dead Sea: the salinity of this sea is so high that it allows a person to float much more easily.

GuilhermeMesquita, Shutterstock.com
We drink fresh water rather than salt water because the salt concentration in salt water is too high for our needs. However, fresh water is not necessarily safe to drink.
To be qualified as drinking water, fresh water must meet certain criteria, which are subject to control and analysis. The water must therefore:
-
be clear;
-
be colourless;
-
be odourless;
-
have a pH of around |7| (be neither too acidic nor too basic);
-
have an acceptable amount of mineral salts;
-
be free from dissolved or suspended undesirable substances (nitrates, pesticides, etc.);
-
be free from toxic substances (arsenic, lead, hydrocarbons, etc.);
-
be free from living pathogenic microorganisms;
-
comply with the maximum concentrations of substances on the list issued by Environment Canada.
Access to drinking water is a major issue for some countries. In the years to come, this will be the case for a growing number of people, given the increase in the world’s population and water depletion. We must do our part and behave responsibly in our consumption of water.
National Centers For Environmental Information. (2009). World Ocean Atlas. https://www.nodc.noaa.gov/OC5/WOA09/woa09data.html