A solution is a homogeneous mixture composed of a solvent and one or more solutes.
An aqueous solution is a solution in which the solvent is water.
Solutions are usually liquids. In a solution, it is impossible to differentiate between the different components of the mixture. Solutions must have only one phase, whether macroscopically (seen with the naked eye) or microscopically (seen through a microscope).
When water and sugar are combined, the resulting sugar water is a solution because it is impossible to distinguish between the components (either with the naked eye or through a microscope).
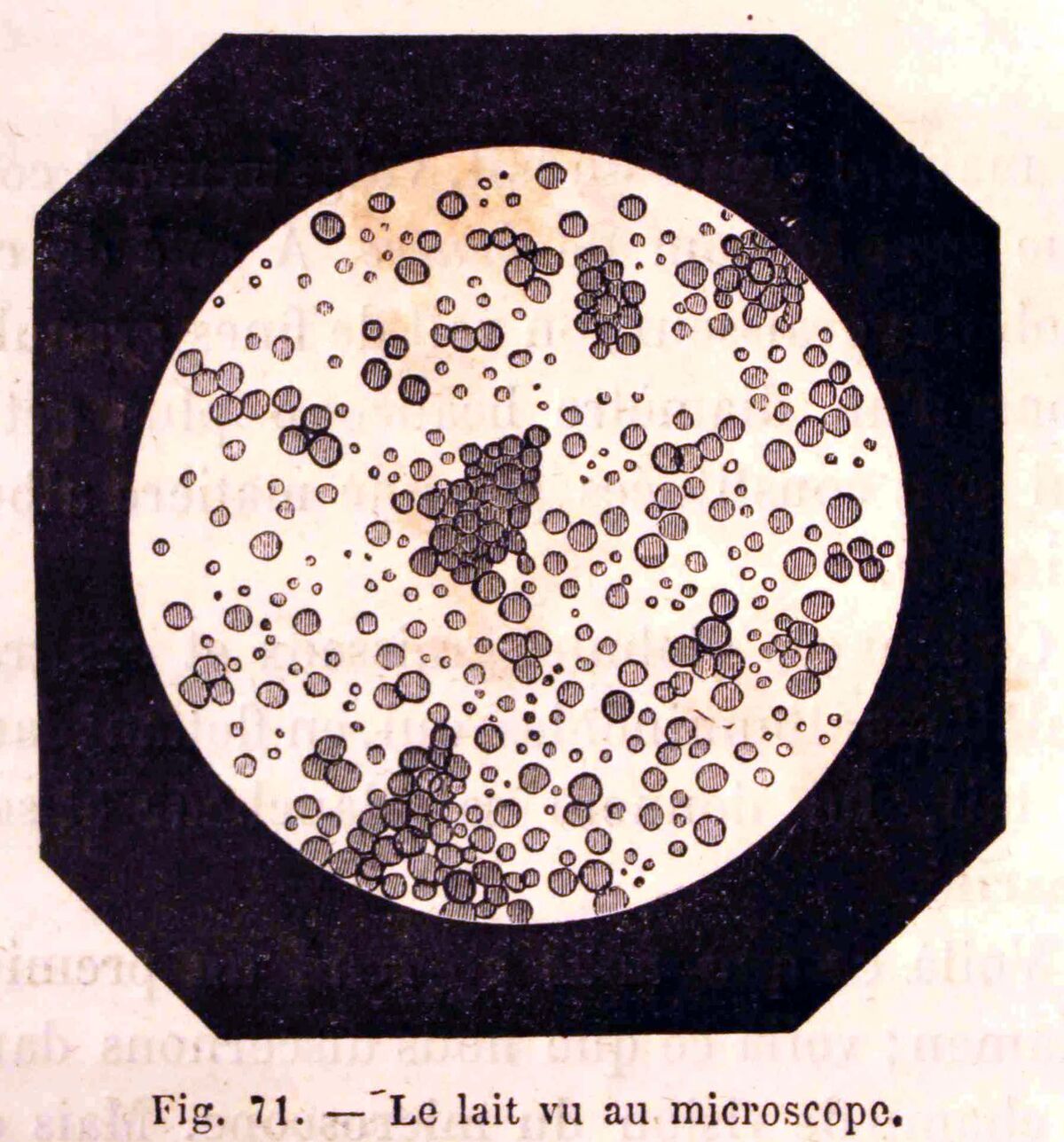
However, milk is not a solution. While it is impossible to distinguish between the components with the naked eye, some of the different elements that make up milk can be observed under a microscope, as seen in the following image.
Solid solutions, or alloys, also exist. An alloy is a homogeneous mixture of several solids.
A bronze medal is a copper and tin alloy.
A solute is the substance that dissolves in a solvent.
In a sugar water solution, the sugar is the solute.
A solvent is the substance found in the largest amount in a solution.
The solute is dissolved in the solvent.
In a sugar water solution, the water is the solvent.
There can be more than one solute in a solution, but only one substance can be the solvent.
The following table shows different examples of solutions based on the different states of matter.
| Solution Classification | |||
| Physical state of the solution | Solute state | Solvent state | Examples |
| gas | gas | gas | air (mixture of mainly nitrogen and oxygen gases) |
| - | - | - | |
| - | - | - | |
| liquid | gas | liquid | oxygen in water |
| liquid | liquid | alcohol in water | |
| solid | liquid | sugar in water | |
| solid | gas | solid | hydrogen in palladium |
| liquid | solid | mercury in gold | |
| solid | solid | carbon in steel | |

