Matter makes up all substances that have a mass and occupy space.
Choose your level.
Matter consists of small particles, called atoms, that are invisible to the naked eye. There are different types of atoms and they can exist on their own or bond with other atoms.
An atom is a particle of matter invisible to the naked eye. It is a building unit of molecules. To find out more about atoms, check out this concept sheet:
A molecule is a group of two or more atoms of the same or different types chemically bonded together. To find out more about molecules, check out this concept sheet:
An element corresponds to one type of atom. The elements are organized in the periodic table of elements. To find out more about the periodic table of the elements, check out this concept sheet:
Atoms, molecules and elements can be seen in the images of the following substances.
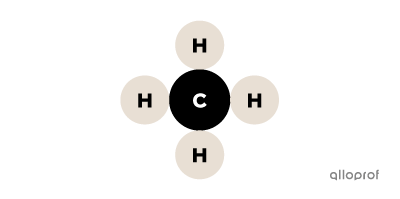
In this image of methane, the following particles can be identified:
-
There are 5 atoms: 1 atom of carbon (C) and 4 atoms of hydrogen (H).
-
There is 1 molecule because it is a group of several atoms bonded together.
-
There are 2 elements because there are 2 types of atoms: hydrogen (H) and carbon (C).
In this image of nitrogen, the following particles can be identified:
-
There are 2 atoms: 2 atoms of nitrogen (N).
-
There is 1 molecule because it is a group of several atoms bonded together.
-
There is 1 element because there is only 1 type of atom: nitrogen (N).

Pour valider ta compréhension à propos de l’organisation de la matière de façon interactive, consulte la MiniRécup suivante.

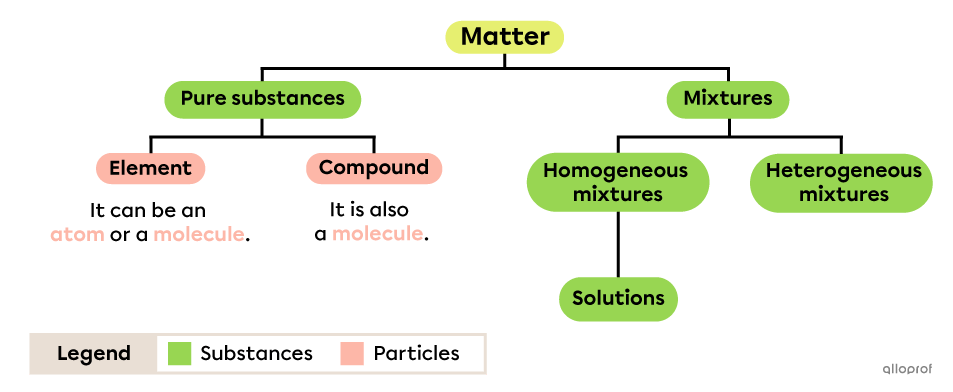
All substances are made up of matter, whether they are pure substances, homogeneous mixtures (solutions) or heterogeneous mixtures.
A pure substance can be made up of different types of particles, either elements or compounds, which are also atoms or molecules. A mixture is made up of different pure substances mixed together.
The organization, properties and behaviour of these particles of matter can be represented and explained using the particle model.
-
A pure substance is made up of a single type of particle.
-
A heterogeneous mixture is made up of several pure substances and it is possible to distinguish several components.
-
A homogeneous mixture is made up of several pure substances and it is impossible to distinguish the components.
-
A solution is a homogeneous mixture in which one substance, called the solute, is dissolved in another, called the solvent.
To find out more about pure substances, mixtures and solutions, check out these concept sheets:
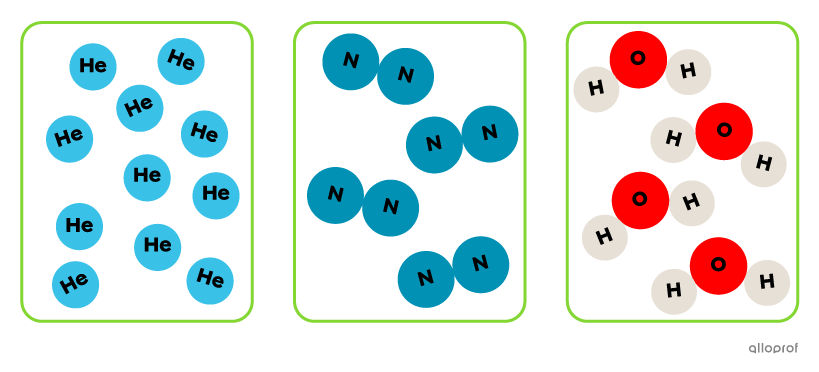
All three substances contain a single type of particle. The substance on the left contains only helium atoms (He), the substance in the centre contains only nitrogen molecules (N2) and the substance on the right contains only water molecules (H2O).
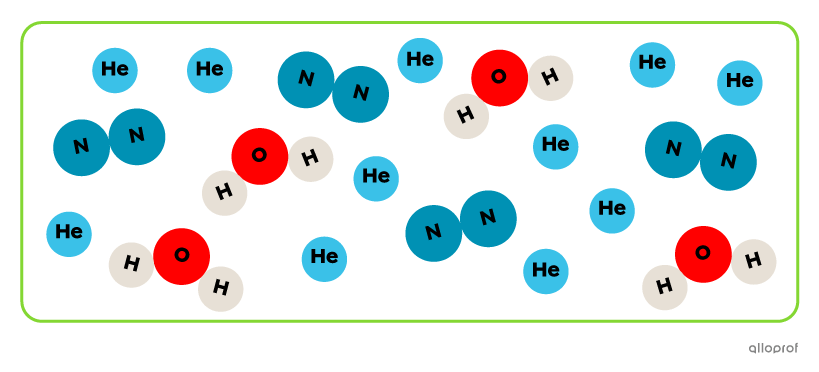
The substance contains different types of particles. It is a mixture of helium (He), nitrogen (N2) and water (H2O).
Whether it's a pure substance or a mixture, a substance is made up of particles. Here are four ways of describing them, depending on their structure.
-
An atom is a particle of matter invisible to the naked eye. It is the building unit of a molecule.
-
A molecule is a particle made up of at least two atoms chemically bonded together.
-
An element is a particle made up of only one type of atom.
-
A compound is a particle made up of several types of atoms.
Une erreur s’est glissée dans cette vidéo.
À 1 min 49 s, la formule chimique est plutôt |HCl,| et non |HCl_2.|
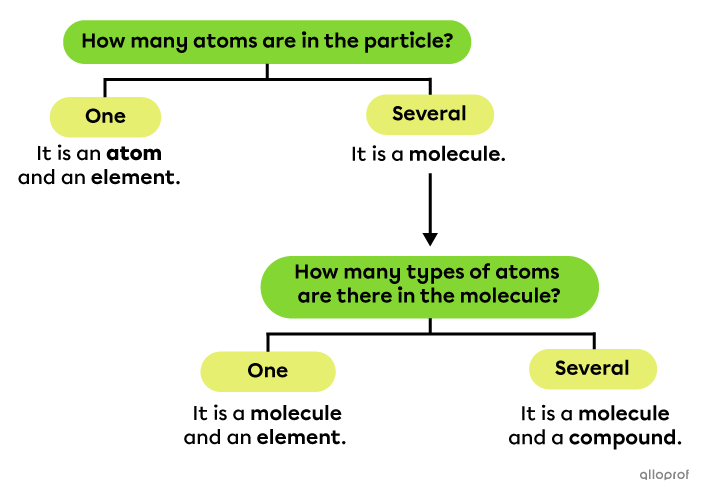
To identify whether a particle is an atom, an element, a molecule and/or a compound, it is helpful to ask the following questions:
Pour valider ta compréhension à propos de l’organisation de la matière de façon interactive, consulte la MiniRécup suivante.

Matter is made up of particles. Here are four ways of describing particles, according to their structure.
-
An atom is a particle of matter invisible to the naked eye. It is the building unit of molecules and compounds in general.
-
An element is a particle made up of only one type of atom.
-
A compound is a particle made up of several types of chemically bonded atoms.
-
A molecule is a particle made up of at least two non-metals chemically bonded together.
The atom can be represented using the following models:
Since atoms, molecules and compounds are present in substances in very large quantities, we use moles to count them and molar mass to measure their mass.
The periodic table organizes all the chemical elements. They are classified according to the composition of their nucleus, their electron configuration and their properties.
To find out more about the periodic table, check out the following concept sheets:
A solution is a homogeneous mixture in which one substance, called the solute, is dissolved in another, called the solvent.
To find out more about solutions, check out the following concept sheets: