The terms heat and temperature are often confused. Although they are both related to thermal energy, heat and temperature are nevertheless two distinct concepts.
Thermal energy is the energy associated with the agitation of particles in a sample.
The thermal energy of a substance depends on:
-
the amount of particles (mass) in the substance
-
the degree of agitation (temperature) of the particles.
The following table shows the variation in thermal energy of the two factors.
|
Factor |
Variation of the factor |
Result |
|---|---|---|
|
Particle quantity (mass) |
The amount of particles increases. |\nearrow| |
Thermal energy increases. |\nearrow| |
|
The amount of particles decreases. |\searrow| |
Thermal energy decreases. |\searrow| |
|
|
Temperature |
The temperature increases. |\nearrow| |
Thermal energy increases. |\nearrow| |
|
The temperature decreases. |\searrow| |
Thermal energy decreases. |\searrow| |
Thermal energy is measured in joules |(\text{J}).| Unlike temperature and mass, there are no instruments for the direct measurement of thermal energy. So it is necessary to resort to indirect methods involving calculations.
Heat is a transfer of thermal energy between two systems with different temperatures.
When two objects with different temperatures are brought into contact, they undergo a change in temperature due to heat (transfer of thermal energy). In short, the initially hot object becomes colder and the initially cold object becomes warmer.
Heat, or thermal energy transfer, always flows from the object with the higher temperature to the object with the lower temperature. As a result of this transfer, the two objects have the same temperature.
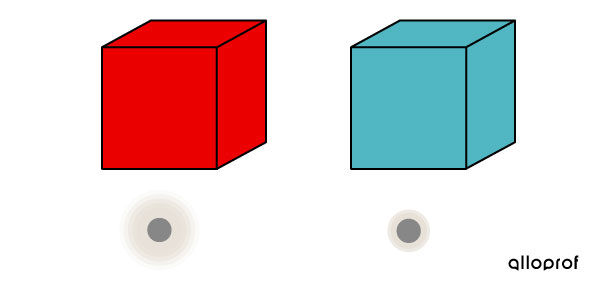
Block 1, with a higher temperature, contains very agitated particles of matter.
Block 2, with a lower temperature, contains less agitated particles of matter.
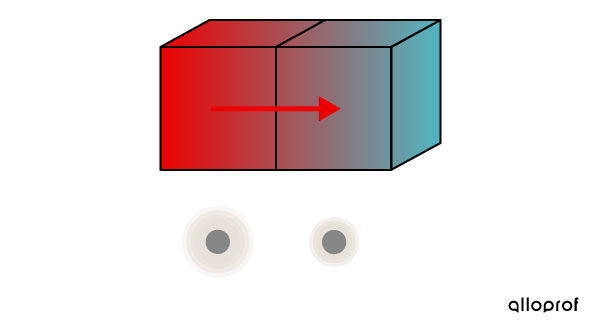
When the two blocks are brought into contact, the more agitated particles in block 1 collide with the less agitated particles in block 2 and transfer thermal energy to them.
The particles in block 1 become less agitated, while the particles in block 2 become more agitated.
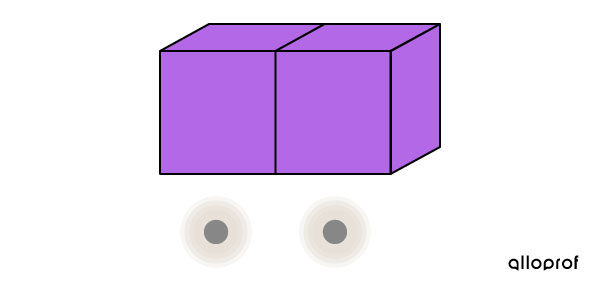
After some time, the transfer of thermal energy is completed. The blocks have the same temperature; therefore, their particles have the same agitation.
To demonstrate the transfer of thermal energy (heat) between two substances, a block of aluminum at |100\ °\text{C}| is immersed in a beaker of water at |5\ °\text{C}|, and the temperature change is observed.
-
Initially, the aluminum block has a temperature of |100\ °\text{C}.|
The water has a temperature of |5\ °\text{C}.|
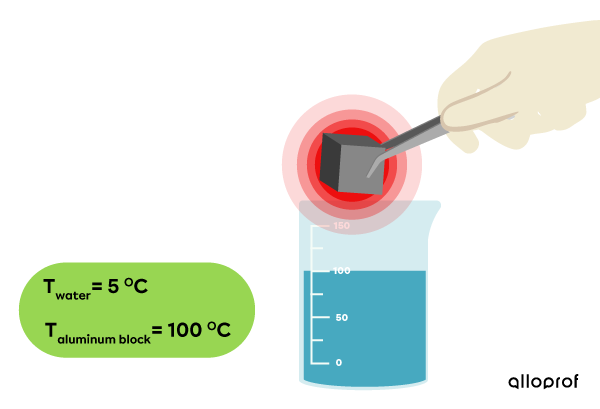
-
The aluminum block is immersed in water and a lid is added to limit heat loss to the ambient air.
Once the aluminum block and the water are in contact, the transfer of thermal energy begins.
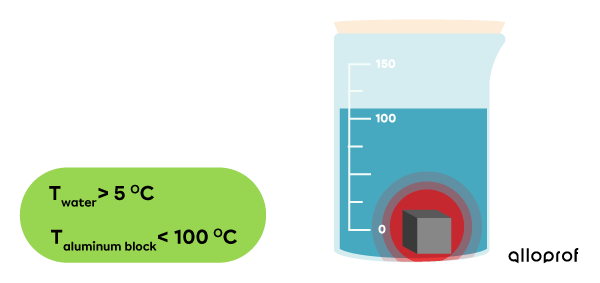
-
We can see that the temperature of the water increases and the aluminum block’s decreases.
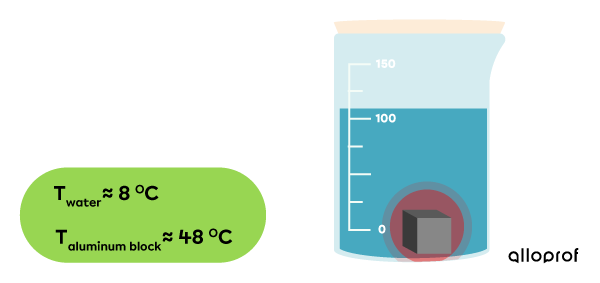
-
After a while, the system’s temperature becomes stable at |10\ °\text{C}.|
The transfer of thermal energy is completed.

In summary, the water has undergone a temperature increase from |5\ °\text{C}| to |10\ °\text{C},| which demonstrates a gain in thermal energy. The aluminum cube has undergone a decrease in temperature going from |100\ °\text{C}| to |10\ °\text{C},| which demonstrates a loss of thermal energy.
In the example, let’s assume that there is no loss of energy to the ambient air.
When you touch a table with a wood top and metal legs, the wood top feels warmer than the metal legs. However, the two parts of the table are components of the same piece of furniture. Both have the same temperature. By understanding the difference between the concepts of temperature and heat, we can explain the observation.
In fact, the table and the legs of the table have the same temperature. However, what we feel when we touch the objects is actually not the temperature, but the heat the objects give off or absorb.
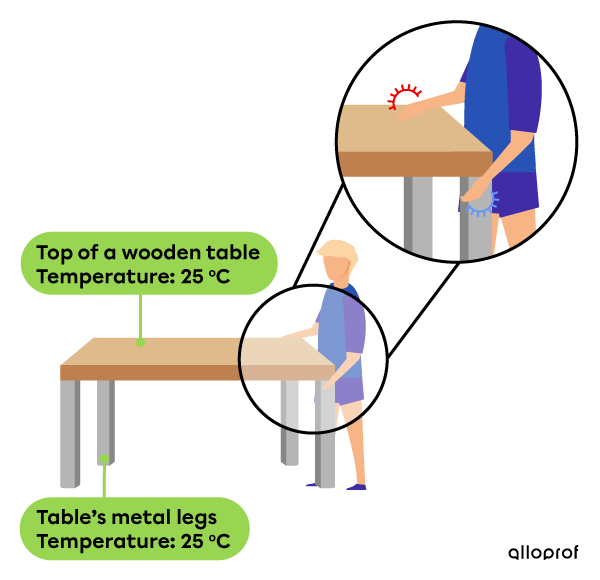
Wood is a thermal insulator. It is why the thermal energy of your hand, which is hotter than the tabletop, travels very little in the wood. It gives off the feeling that the wood is warmer than the metal. On the other hand, when touching a table’s metal leg, your hand transfers a greater amount of thermal energy. The metal leg absorbs heat because metal is a thermal conductor. It feels cold on your hand because the piece of metal absorbs more heat.
Temperature is the measure of the degree of agitation of the atoms and molecules of a substance.
Temperature is a non-characteristic property of matter. It is used to quantify the agitation of the particles in a substance. The more the particles are agitated, the higher the temperature.
Temperature is measured with a thermometer in units of degrees Celsius |(°\text{C}),| degrees Fahrenheit |(°\text{F})|, and the Kelvin |(\text{K}).|
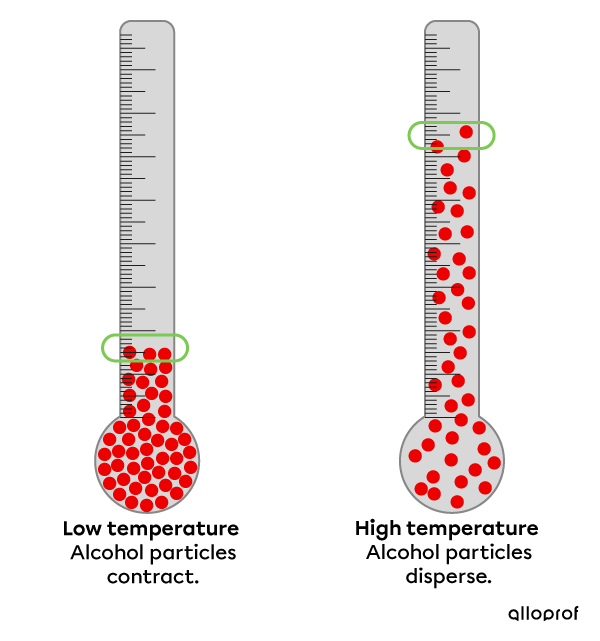
The alcohol thermometer uses the principle of thermal expansion to measure the temperature of a substance.
When the temperature is low, the degree of agitation is lower and the alcohol particles are closer together.
When thermal energy is increased, the alcohol particles become more agitated and disperse.
The scale on the glass tube provides a numerical reading of the temperature.