The separation of mixtures makes it possible to isolate or separate certain components of the mixtures in which they are found.
To obtain a pure substance, it is often necessary to separate this substance from all the other ones. Mixtures can be separated by physical means, which will be discussed in this concept sheet. The choice of technique varies depending on the mixture, the substance to be separated from the rest of the mixture and the phases making up the mixture.
The techniques that are used most often are the following.
Evaporation is a process by which the liquid part of a mixture is removed by turning it into gas.
This can be done by allowing the liquid component of the mixture to evaporate naturally at room temperature.The process can also be sped up by heating the mixture. Evaporation is used to recover the solid part of a heterogeneous mixture or the solid solute of a solution. Furthermore, evaporation allows the solute of a solution to be concentrated in a smaller volume of solvent, as shown in the following image.
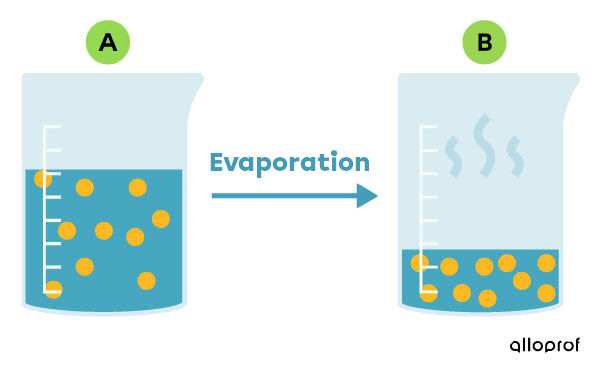
To find out how to practice the evaporation technique in the laboratory, refer to the concept sheet about techniques for separating mixtures (lab).
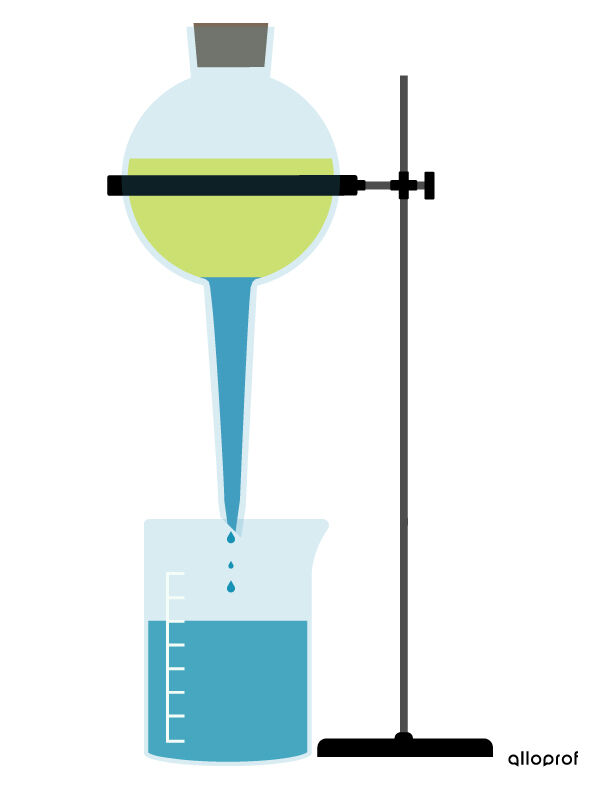
Decantation is a process that separates immiscible substances that do not have the same density.
When the components to be separated are liquid, they are put into a separatory funnel and left untouched. The liquid which has the greater density then moves towards the bottom of the funnel. The liquid which has the lower density, on the other hand, is pushed upwards. When the 2 phases are quite distinct, the 2 liquids can be separated in this way.
This technique can be used to separate a mixture of water and oil. As seen in the following image, oil floats on water because oil has a lower density than water.
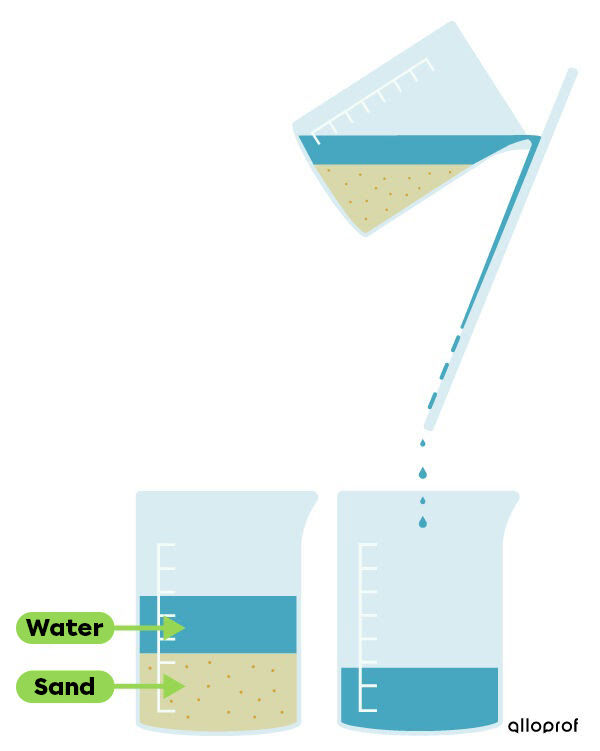
Decantation can also help to separate solid particles suspended in a liquid, which is often called sedimentation. During sedimentation, the suspended particles stop moving and settle to the bottom of the container, due to the effect of gravity. The deposit is then called sediment. Once the suspended particles have settled to the bottom of the container, a glass rod is used to pour the liquid into another container. This technique ends with the liquid in one container and the solid part in another.
This technique can be used with a mixture of water and sand. The mixture is left to stand for some time. The sand then settles to the bottom of the container. The water is then transferred, using a glass rod, into another container.
To find out how to perform the decantation technique in the lab, refer to the concept sheet about techniques for separating mixtures (lab).
Filtration is a technique which makes it possible to separate the components of a mixture when one of the components is in the liquid phase and the other is in the solid phase.
To do this, a filter is used. This filter retains solid particles that are larger than the pores (holes) of the filter. The liquid that passes through the filter is called the filtrate and the solid that is collected in the filter is called the residue.
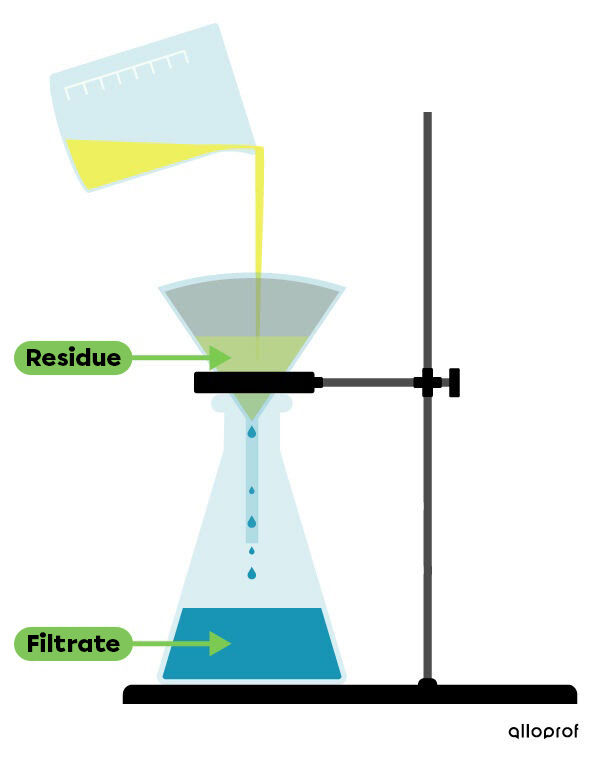
To learn how to practice the filtration technique in the laboratory, consult the concept sheet about the separation techniques of mixtures (lab).
Distillation is a mixture separation technique used to separate the components of a liquid homogeneous mixture or of a heterogeneous mixture involving at least one liquid phase.
For this technique, the boiling point is used. The mixture is heated until the boiling point of one of the components is reached, which then evaporates. The vapour is then collected and condensed in another container. While the first liquid evaporates (distillate), the second does not reach its evaporating temperature and remains in the initial container (residue) in its liquid form.
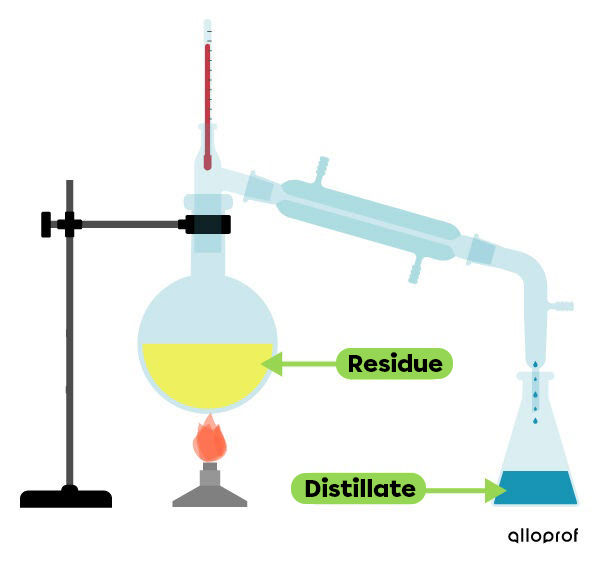
Using this technique, a mixture of alcohol and water can be separated. Alcohol has a lower boiling temperature than water, so it evaporates first. The alcohol vapour is collected and cooled. This condensation makes it possible to recover the alcohol (distillate) in another container. The water (residue) remains in the original container.
To learn more about the laboratory distillation technique, consult the concept sheet about mixing separation techniques (lab).
The technique of sieving consists of separating the components of a mixture of solid substances using a sieve.
A sieve is an instrument that looks like a wire mesh, like the strings of a tennis racket. The space between the wires is more or less large, which allows the smaller particles of the mixture to pass through and traps the larger particles. It is then possible to separate the mixture components according to the size of their particles.

Konstantin Kolosov, Shutterstock.com
A mixture of fine sand and pebbles can be separated using a sieve. Simply pass the whole mixture through the sieve. The pebbles will remain in the sieve and the fine sand will pass through.
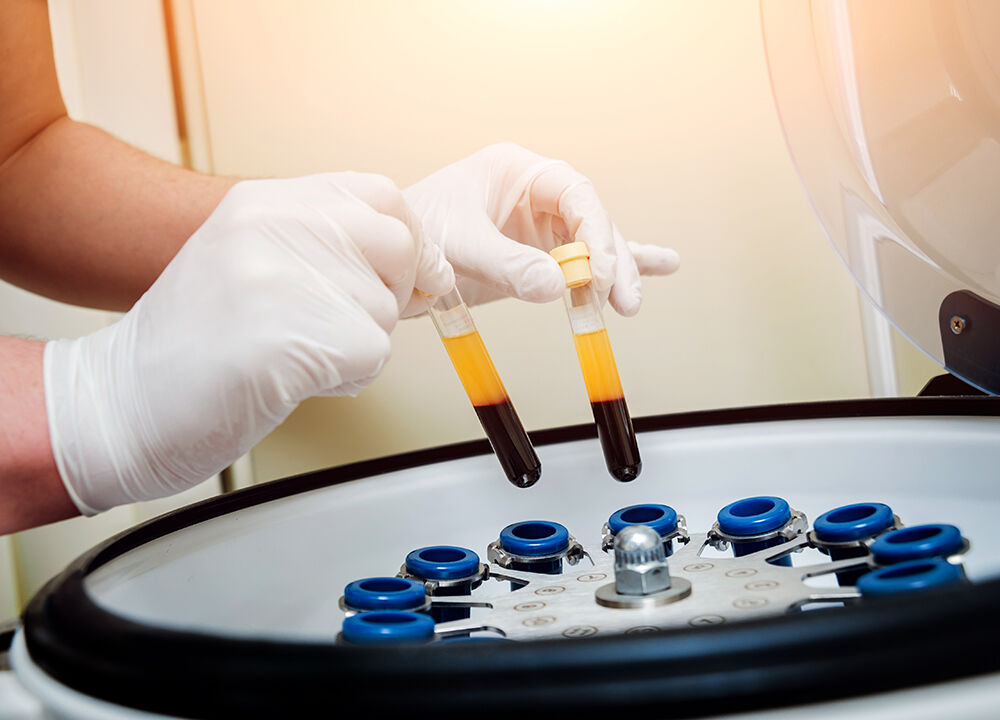
Centrifugation is a separation technique which, by the action of centrifugal force, makes it possible to separate 2 to 3 phases of a mixture.
The mixture is driven in a very fast rotating movement. The heavier solid particles are then pushed towards the container’s walls under the action of centrifugal force, while the lighter particles and liquids remain on the surface, referred to as the supernatant. The apparatus used to perform centrifugation is called a centrifuge.
Red blood cells can be separated from blood plasma using a centrifuge.
Ktsdesign, Shutterstock.com
Roman Zaiets, Shutterstock.com
The operating principle is the same as for a salad spinner. We place the salad in the spinner and turn everything very quickly. The salad leaves stick to the wall of the basket while the water sticks to the sides of the container. When everything stops spinning, the water falls and it is collected at the bottom of the basket.
To know how to perform the centrifugation technique in the lab, refer to the concept sheet on the techniques of separation of mixtures (lab).
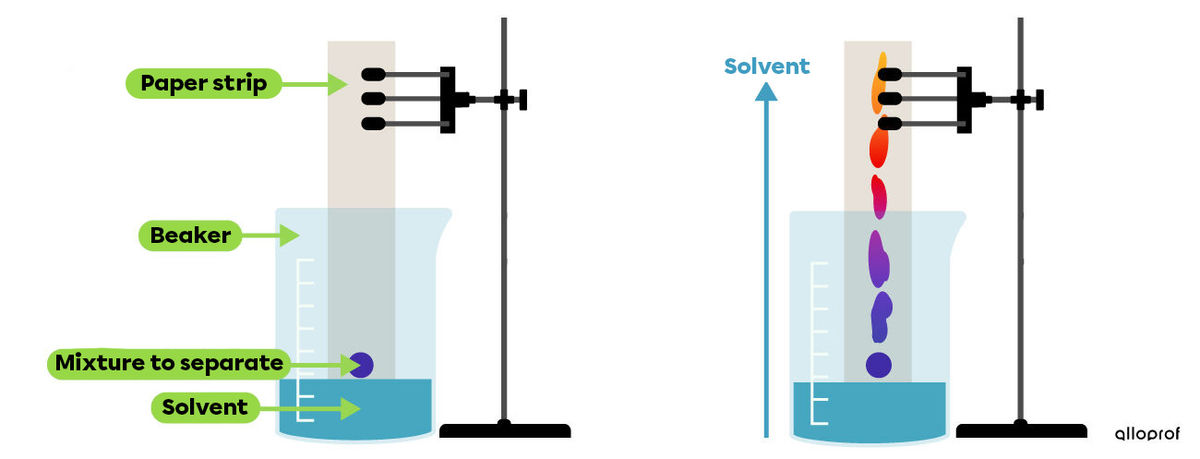
Paper chromatography makes it possible to separate the components of a mixture thanks to their different migration speeds.
To perform this technique, hold paper strips vertically and immerse their base in a solvent. The solvent can be water or alcohol. The mixture that is to be separated is placed at the base of the paper strip.
The liquid then rises along the paper strip through capillary action and carries the various components of the mixture with it.
Each component is carried at a different speed. The different substances separate as they travel along the paper. The substances that are separated, often molecules, finally appear as colored spots.

Ggw, Shutterstock.com
In some cases, the mixture to be separated is dissolved directly in the solvent, rather than placing it on the paper strip. The solvent then carries the mixture with it as it rises along the strip of paper.
There are more complex techniques for separating mixtures. These require, among other things, the addition of reagents to initiate a chemical reaction (precipitation).
Precipitation consists in forming a heterogeneous phase within another phase. If the presence of certain ions in a solution is suspected, another ion can be added to form a solid substance with them. If the desired ion is indeed present, a solid substance will appear. This substance can then be filtered and recovered. Precipitation is a chemical means used to separate mixtures.
If we want to recover the lead ions in a solution of lead nitrate (|Pb(NO_{3})_{2}|), a solution containing iodine can be added. Lead binds with iodine to form lead iodide (|PbI_{2}|), which is a yellow powdery solid.
