This concept sheet explains the procedures to be followed to prepare a solution with a required concentration.
A solution is a mixture composed of a substance present in a small quantity (the solute), dissolved in another substance present in a larger quantity (the solvent). When the solvent is water, this mixture is called an aqueous solution.
There are two methods to prepare these two solutions: dissolution or dilution.
In a dissolution, take the solute and dissolve it in the solvent to obtain the required solution.
In order to prepare the solution at the required concentration, it is necessary to know the volume of the solution to be prepared and the quantity of solute necessary to prepare it. Generally, the volume is determined by the volumetric flask in which the solution is prepared. However, mass is usually not mentioned and it is, therefore, necessary to calculate it before starting the procedure.
How much solute must be measured to prepare a solution of |\small 12 \: \text {g/L}| in a |\small 100\: \text {ml}| volumetric flask?
The first step is to identify the variables in this case:
||\begin{align}C &= 12\: \text{g/L} \\ m &=x\: \text{} \\V &=100\: \text{mL} = 0.100\:\text{L}\end{align}||
Using the concentration formula, the amount of solute to add can be calculated.
||\begin{align} \displaystyle C=\frac{m}{V} \quad \Rightarrow \quad m &=
C\times V\\ \\
&= \displaystyle 12\: \text{g/L} \times 0.100\:\text{L}\\ \\
&= 1.2 \:\text{g} \end{align}||
Therefore, it will be necessary to measure |1.2 \:\text{g}| of solute to prepare this solution.
- Solid to dissolve
- Weighing dish
- Spatula
- Beam balance
- Distilled water
- Graduated cylinder
- Volumetric flask
- Rubber stopper
- Lab coat
- Safety glasses

1. Calculate the amount of solute needed to prepare the required solution.
2. Weigh the weighing dish with the beam balance, and record its mass.
3. Calculate the total mass of the solute with the weighing dish.
If the weighing dish has a mass of |\small 2.49 \text { g}|, and |\small 1.2 \text { g}| of solute is to be added, then the total mass of the weighing dish with the solute will be calculated as follows:
|\small 2.49 \text { g} + \small 1.2 \text { g} = \small 3.69 \text { g}|.
It is therefore necessary to move the sliders of the balance to |\small 3.69 \text { g}|. This will represent the mass of the weighing dish with the solute in it.
4. Using the weighing dish and the beam balance, add the solute until the pointer is aligned with the zero mark of the balance.

5. Add the solvent to the volumetric flask to obtain about half the total volume of the solution.

Do not immediately put the total volume of the solvent in the volumetric flask, as the final solution will exceed 100 mL since the solute slightly increases the volume of the solution.
6. Pour the solution into the volumetric flask.

7. Shake until completely dissolved.
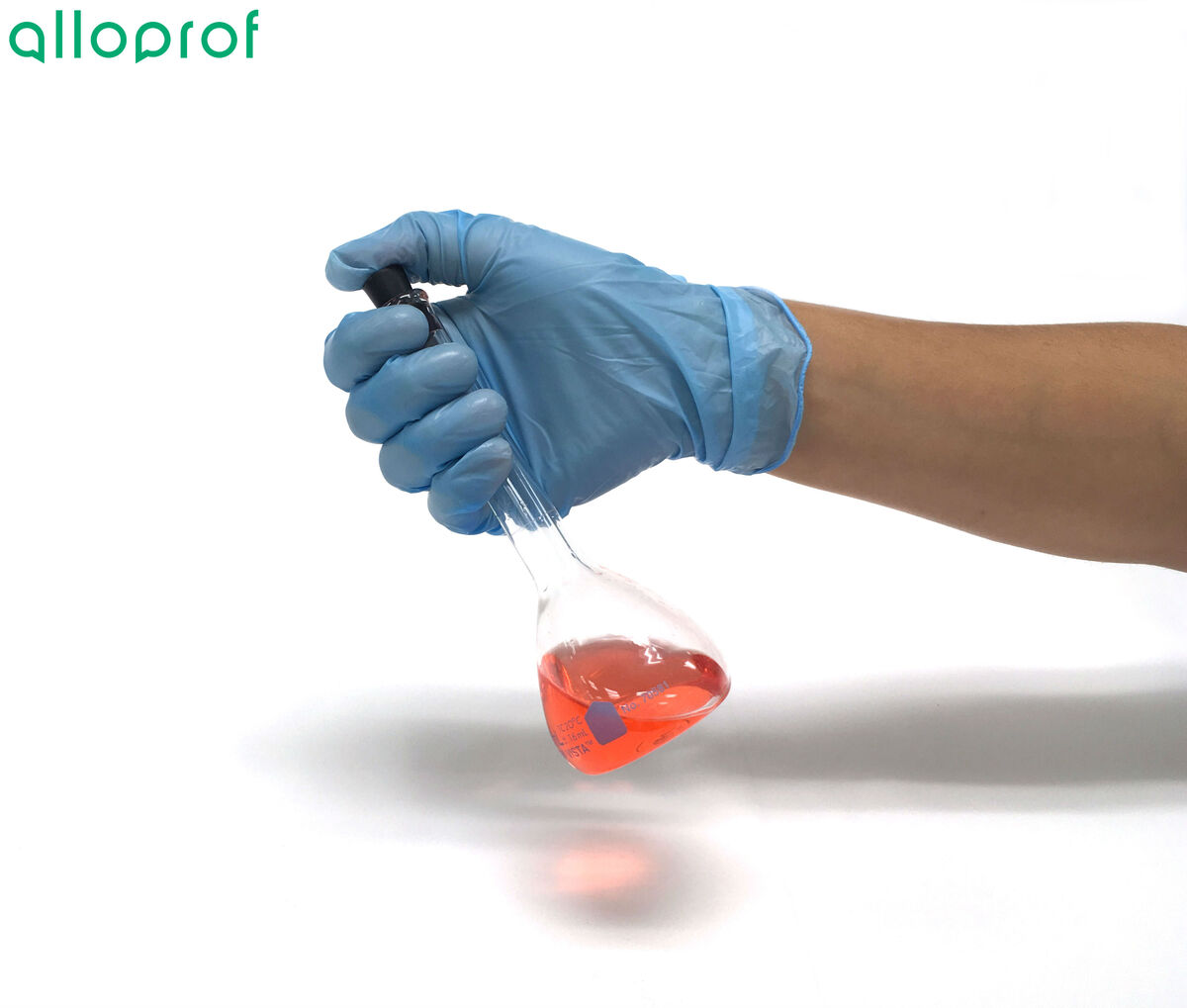
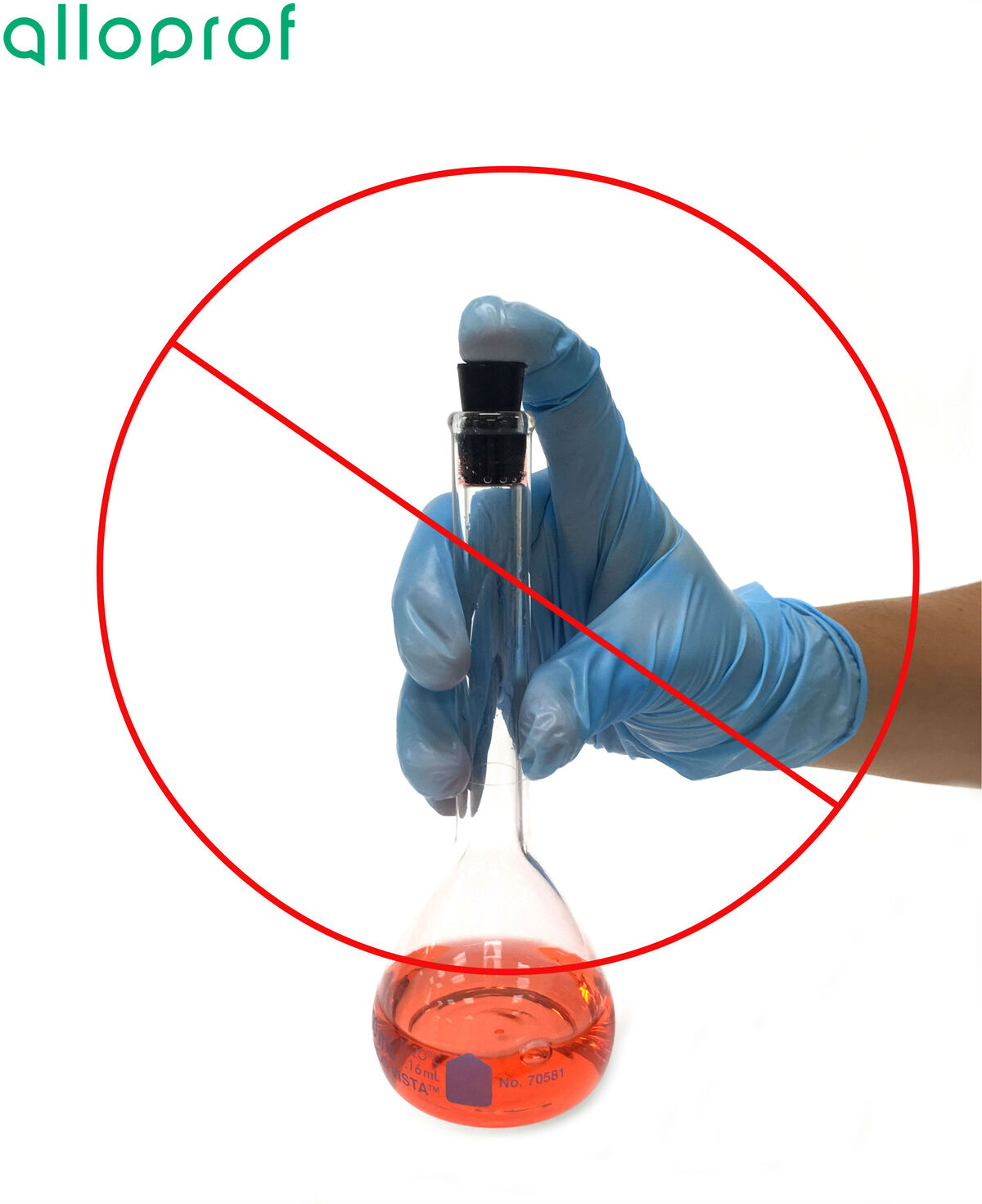
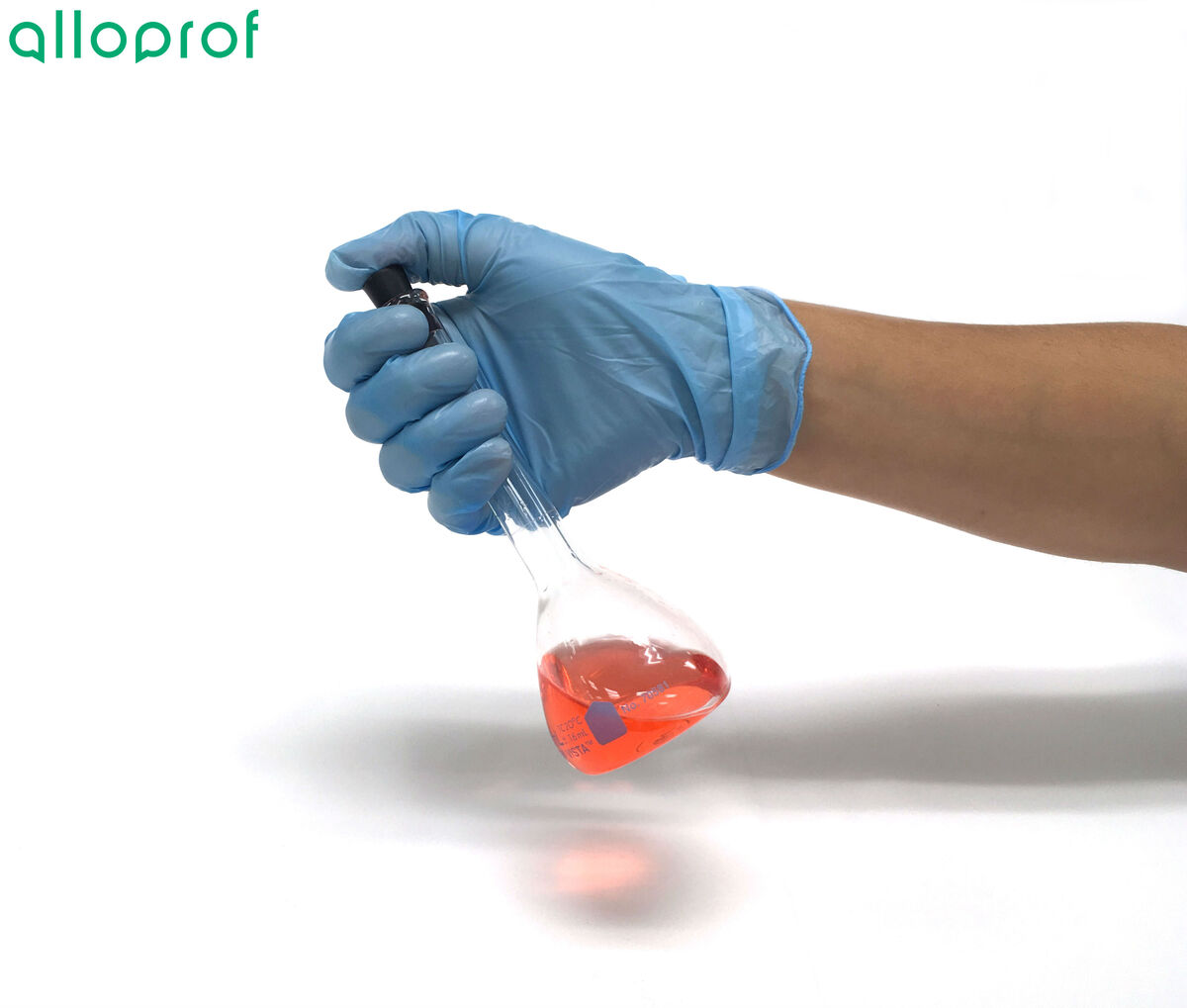
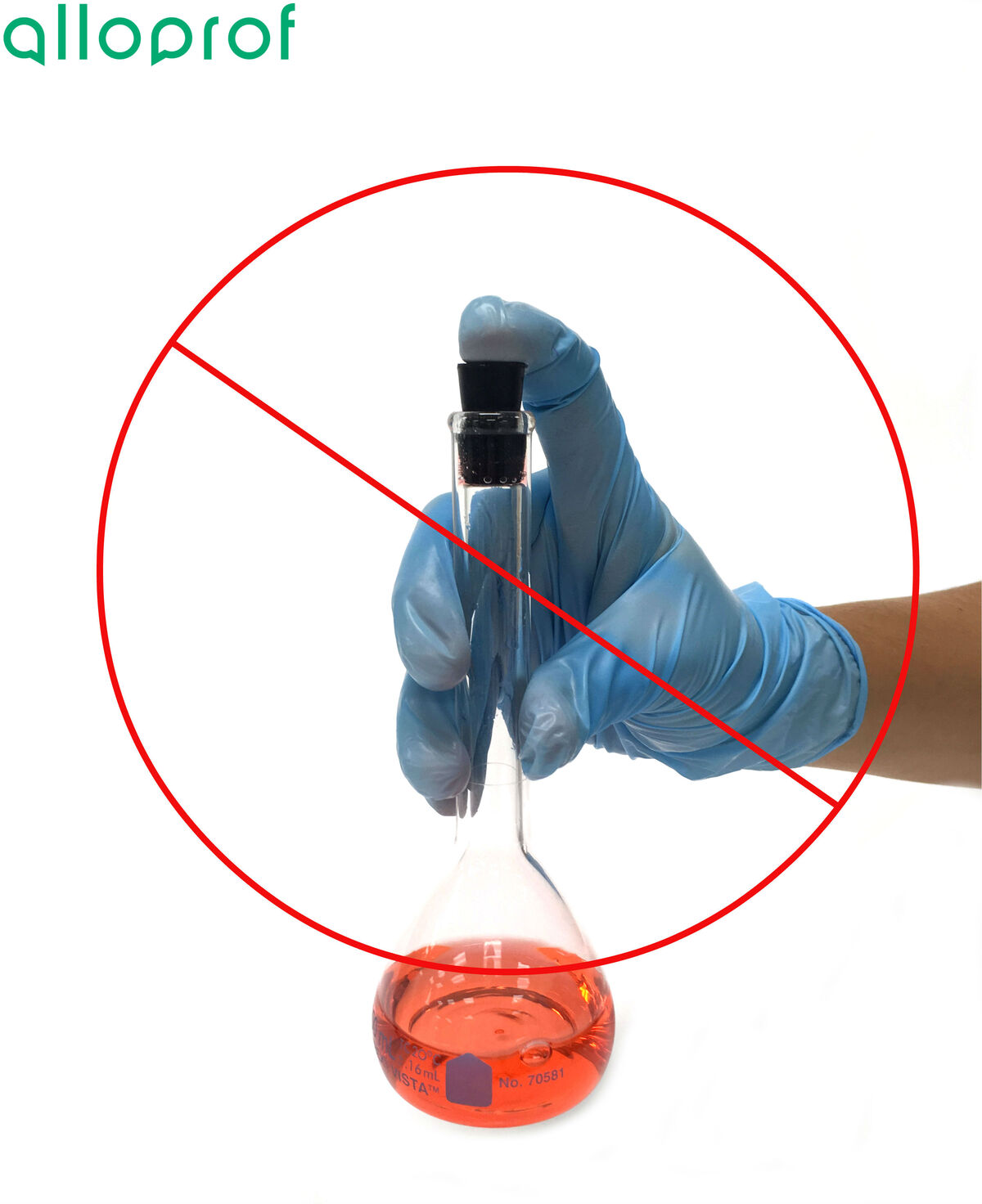
To prevent the mixture from spilling, it is recommended to hold the volumetric flask by the upper part while placing your thumb on the stopper. This will ensure a tighter seal of the stopper on the volumetric flask.

If the solute has not completely dissolved, add a small amount of solvent without exceeding the maximum volume and stir again.
8. Add solvent until obtaining the maximum total solution volume. Using the dropper can be an option to achieve the required precision more conveniently.

9. Shake again.
10. Clean and store equipment.
The calculations were already done at the beginning of the experiment. The experimental data should, therefore, be presented in the form of a results table.
Preparing a Solution by Dissolution
| | Solution |
| |m| | |\text {1.2 g}| |
| |V| | |\text {0.100 L}| |
| |C| | |\text {12 g/L}| |
The prepared solution can then be used in another experiment. In some cases, to check the quality of the preparation made, a colourimetric test or a comparison with controls may be required to ensure that the concentration prepared is correct.
In a dilution, the solution must be taken and solvent added to it to reduce its concentration.
To prepare a diluted solution, it is first necessary to determine how much of the initial solution will be used to prepare the new diluted solution. To achieve this, it is important to know the initial and final concentrations of the solutions, as well as the new solution’s final volume.
How much of an initial solution with a concentration of |\small 100 \: \text {g/L}| should be measured to prepare a solution with a concentration of |\small 20 \: \text {g/L}| in a volumetric flask of |\small 250 \: \text {mL}| ?
The first step is to identify the variables in this case:
||\begin{align}C_{1} &= 100\: \text{g/L} & &\quad & C_{2} &= 20\:\text{g/L}\\
V_{1} &= x & & & V_{2} &= \: 250 \: \text{ml} \:= \: 0.250 \: \text{L}\\
\end{align}||
The amount of solute to add can be calculated by using the concentration formula:
||\begin{align} C_1\times V_1=C_2\times V_2 \quad \Rightarrow \quad V_1 &=\displaystyle\frac{C_2 \times V_2}{C_1}
\\ \\
&= \displaystyle\frac{20\: \text{g/L} \times 0.250 \:\text{L}}{100\: \text{g/L}}\\ \\
&= 0.05 \:\text{L} = 50 \:\text{mL}\end{align}||
It will, therefore, be necessary to measure |50 \:\text{mL}| of the initial solution to |\small 100 \: \text {g/L}| to prepare this solution.
- Initial solution
- Graduated cylinder
- Distilled water
- Volumetric flask
- Rubber stopper
- Lab coat
- Safety glasses

1. Calculate the amount of initial solution needed to prepare the required solution.
2. Measure the quantity calculated in the previous step using a graduated cylinder.

3. Pour the volume measured in step 2 into the volumetric flask.

4. Add solvent until the maximum total solution volume is obtained. Using the dropper can be an option to achieve the required precision more conveniently.

5. Shake to make the mixture homogeneous.

To prevent the mixture from spilling, it is recommended to hold the volumetric flask by the upper part while placing your thumb on the stopper. This will ensure a tighter seal of the stopper on the volumetric flask.

6. Clean and store the equipment.
The calculations have already been made before starting the experiment. Therefore, simply present the important experimental values in the form of a table of results.
Preparing a Solution by Dilution
| | Solution |
| |C_1| | |100 \text {g/L}| |
| |V_1| | |\text {0.050 L ou 50 mL}| |
| |C_2| | |\text {20 g/L}| |
| |V_2| | |\text {0.50 L ou 250 mL}| |
Colourimetry or comparison with controls are two techniques that can be used to validate the quality of the approach.