A/Z notation is used to distinguish isotopes of the same element. It displays the symbol of the element (|X|), its atomic number (|Z|), and its mass number (|A|).
A/Z notation has the following structure:
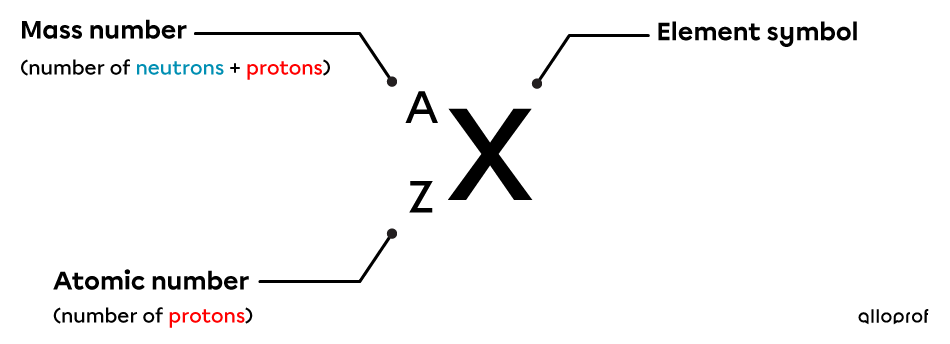
The following table summarizes the information that A/Z notation provides regarding the composition of atoms in terms of the number of neutrons, protons, and electrons. Two isotopes of carbon, namely carbon |12| and carbon |13|, are chosen to illustrate their composition.
|
Carbon 12 |
Carbon 13 |
|
|---|---|---|
|
A/Z isotope notation |
|_{6}^{12}\text{C}| |
|_{6}^{13}\text{C}| |
|
Mass number |
|12| |
|13| |
|
Atomic number |
|6| |
|6| |
|
Number of neutrons |
|6| |
|7| |
|
Number of electrons |
|6| |
|6| |
We see that the atomic number (|Z|) from |2| carbon isotopes is |6|, as they both have |6| protons. In fact, the number of protons of an atom never changes. Carbon isotopes, therefore, always have |6| protons in their nucleus.
Also, because atoms are neutral, they both have the same number of electrons as protons. So there are |6| protons and |6| electrons. Positive charges and negative charges cancel each other out.
On the other hand, the mass number (|A|) varies (either |12|, or |13|), as the isotopes of the same element do not have the same number of neutrons (here, |6| and |7| neutrons).
The A/Z notation for an oxygen isotope is as follows: |_{8}^{17}\text{O}|.
What is the composition of this isotope (number of protons, neutrons, and electrons)?
To solve the problem, first identify the information displayed in A/Z notation for the isotope |_{8}^{17}\text{O}|, that is:
-
mass number: |A = 17|;
-
atomic number: |Z = 8|.
Then, analyze that:
-
the number of protons corresponds to the atomic number, or |8|;
-
the atom is neutral so the number of electrons is equal to the number of protons, which is |8|;
-
the number of neutrons corresponds to the mass number minus the atomic number:
|\begin{align} N &= A-Z \\ N &=17-8 \\ N &=9 \end{align}|
Therefore, the isotope |_{8}^{17}\text{O}| comprises |8| protons, |8| electrons, and |9| neutrons.
What is the A/Z notation for magnesium-|26|?
To solve the problem, we need to find the value of |X,| |A|, and |Z.|
-
In the periodic table of elements, the (|X|) symbol of magnesium is |\text{Mg}.|
-
In addition, its atomic number (|Z|) is |12.|
-
Finally, in the problem statement, the value of the mass number (|A|) is given. It is |26.|
Thus, the notation |_{12}^{26}\text{Mg}| is the A/Z notation for magnesium-|26.|
An isotope of palladium consists of |46| protons, |46| electrons, and |62| neutrons. What is the A/Z notation for this isotope?
To solve the problem, find the value of |X|, |A|, and |Z|.
-
In the periodic table of elements, the (|X|) symbol for palladium is |\text{Pd}.|
-
In addition, palladium’s atomic number (|Z|), which corresponds to its number of protons, is |46|. This can be seen by looking at the periodic table or by referring to the statement.
-
The mass number (|A|) corresponds to the sum of the number of protons and the number of neutrons:
|\begin{align}A &= 62 + 46\\ A &= 108 \end{align}| -
Finally, as the number of protons is equal to the number of electrons, we can see that the atom is neutral.
Thus, the |_{46}^{108}\text{Pd}| notation is the A/Z notation for the palladium isotope described in the statement.
Sometimes the distribution of electrons is added to A/Z notation.
The A/Z notation for oxygen-|16| with its electronic distribution is noted as follows: |_{8}^{16}\text{O}:2e^{-},6e^{-}.|
A/Z notation can also be applied to ions. The only difference with neutral atoms is that the charge is indicated at the top right of the symbol, for example |_{11}^{23}\text{Na}^+| or |_{17}^{35}\text{Cl}^-|.
To illustrate this, the following table shows the composition of carbon-|12| when it is neutral, positive, or negative.
| Atom / Ion | Number of neutrons | Number of protons | Number of electrons |
|---|---|---|---|
| |_{6}^{12}\text{C}| | |6| | |6| | |6| |
| |_{6}^{12}\text{C}^+| | |6| | |6| | |5| |
| |_{6}^{12}\text{C}^-| | |6| | |6| | |7| |
All carbon atoms have |6| protons. When the charge of carbon changes, the number of protons always remains the same; the number of electrons is the varying element. When there are more electrons than protons, the ion is negative (e.g., charge |1-| , |2-| , |3-| , etc). When there are fewer electrons than protons, the ion is positive (e.g., charge |1+|, |2+|, |3+|, etc).