Noble gases, also called inert gases, are very stable elements because the last electron shell of their atoms contains the maximum possible number of electrons. These atoms do not tend to lose or gain electrons nor to bond with other atoms.
The atoms of other elements have an incomplete last electron shell. They are less stable than noble gases. In order to become more stable, an atom can lose or gain electrons and take on the electron configuration of a noble gas. The atom becomes an ion, which is a process known as ionization.
An ion is an electrically charged particle that forms when an atom loses or gains electrons.
To determine the charge of an ion, the sum of charges has to be calculated. Simply add up the number of positive charges (protons) and the number of negative charges (electrons).
-
If the sum of charges is zero |(0),| the atom is neutral and it is not an ion.
-
If the sum of charges is positive |(+),| it is an ion, or more specifically, a cation.
-
If the sum of charges is negative |(-),| it is an ion, or more specifically, an anion.
The charge of an ion is shown to the right of its chemical formula as a superscript. When the charge is |+1| or |-1,| it is not necessary to write the |1.| Simply write the |+| sign or the |-| sign as a superscript.
-
A cation is a positively charged ion.
-
An anion is a negatively charged ion
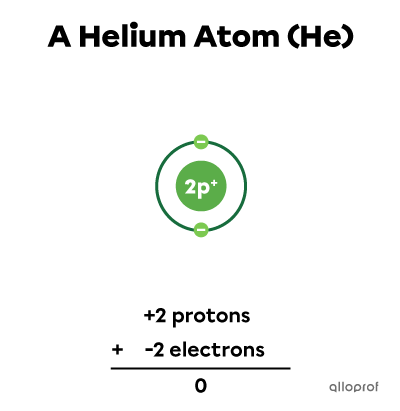
The sum of charges is |0.| This is a helium atom |(\text{He}).|

The sum of charges is |+1.| This is a sodium cation |\text{Na}^{+}.|

The sum of charges is |-2.| This is a sulphur anion |\text{S}^{2-}.|
-
The duet rule is the tendency of certain atoms to take on the electron configuration of helium |(\text{He}),| a noble gas that has two valence electrons.
-
The octet rule is the tendency of certain atoms to take on the electron configuration of a noble gas that has eight valence electrons.
The lithium ion |(\text{Li}^{+})| respects the duet rule. By losing one electron, it takes on the electron configuration of helium |(\text{He}).|
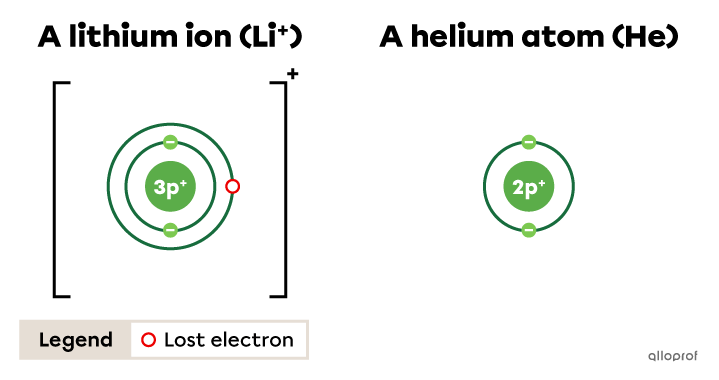
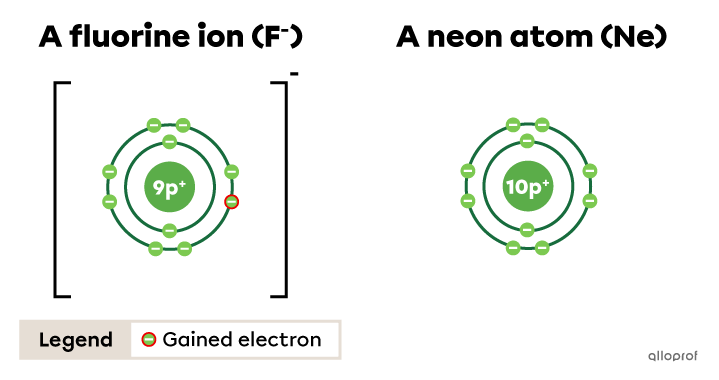
The fluorine ion |(\text{F}^{-})| respects the octet rule. By gaining one electron, it takes on the electron configuration of neon |(\text{Ne}).|
The duet and octet rules can be used to predict the possible chemical bonds (EST) between different atoms. They are also used to predict the electron configuration and charge obtained by an ion.
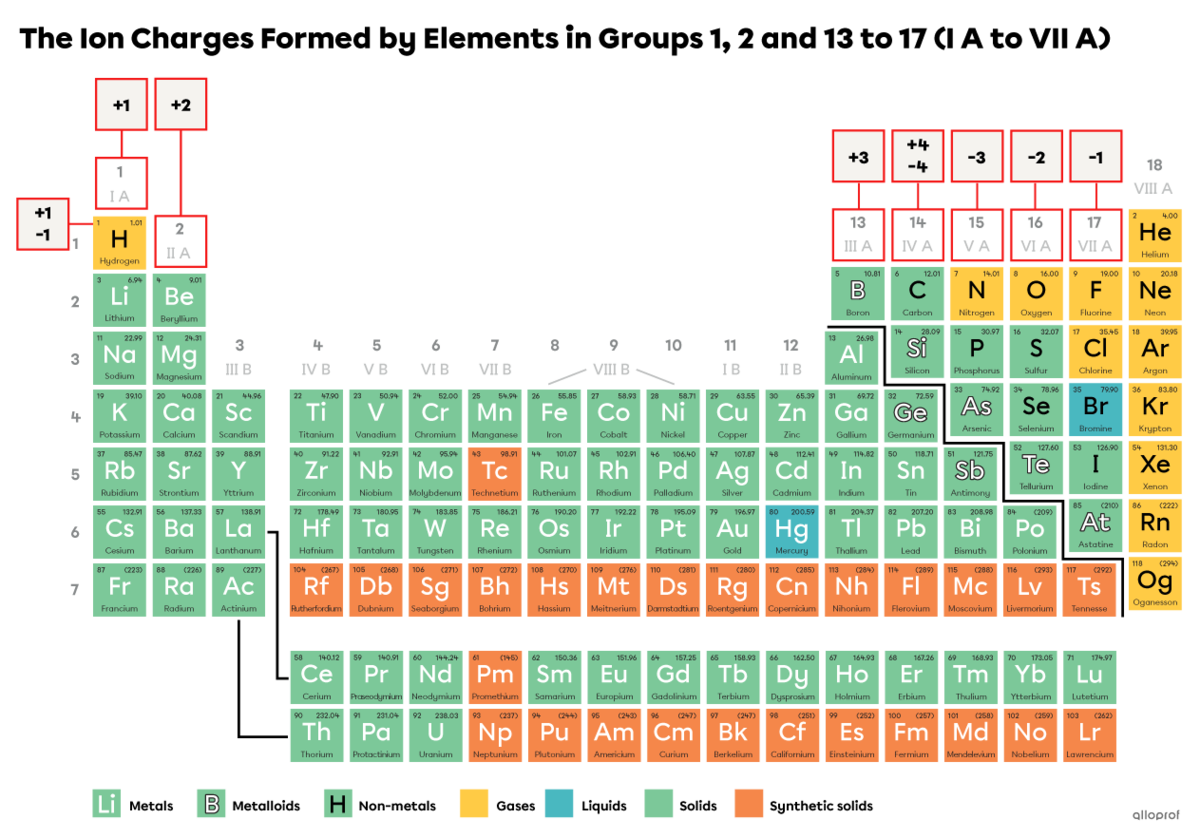
Generally, an atom becomes an ion by taking on the electron configuration of the noble gas closest to it in the periodic table of elements.
In the periodic table, it takes two jumps to the right to go from the sulphur square |(\text{S})| to the closest noble gas, which is argon |(\text{Ar}).| These two jumps represent a gain of |2| electrons by the sulphur.
The sulphur ion |(\text{S}^{2-})| gained |2| electrons to obtain the electron configuration of an argon atom.
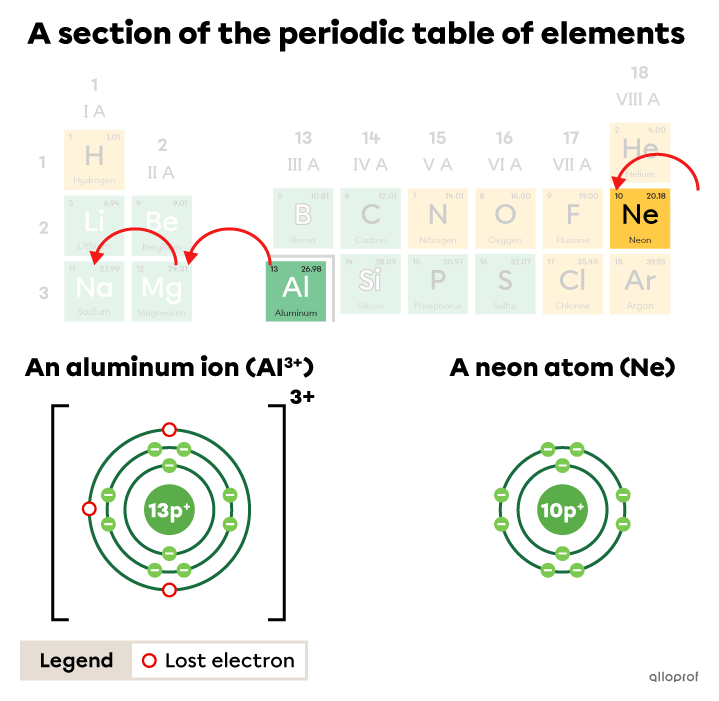
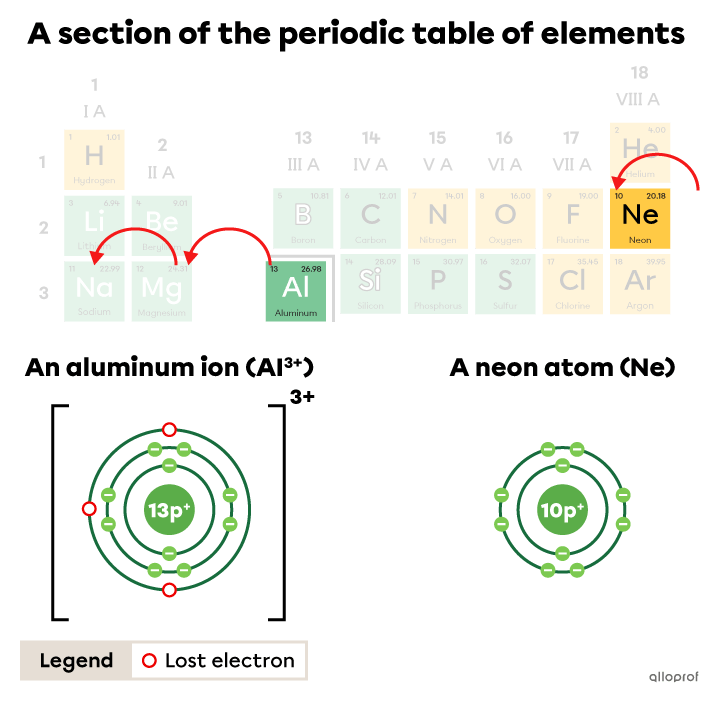
In the periodic table, it takes three jumps to the left to go from the aluminum square |(\text{Al})| to the closest noble gas, which is neon |(\text{Ne}).| These three jumps represent a loss of |3| electrons by the aluminum.
The aluminum ion |(\text{Al}^{3+})| lost |3| electrons to obtain the electron configuration of a neon atom.
-
Hydrogen does not belong to any of the groups. It can lose one electron and obtain a |+1| charge or gain one electron and obtain a |-1| charge.
-
Alkali metals, which are metals in Group 1 (I A), tend to lose one electron and obtain a |+1| charge.
-
Alkaline-earth metals, which are metals in Group 2 (II A), tend to lose two electrons and obtain a |+2| charge.
-
Elements in Group 13 (III A), tend to lose three electrons and obtain a |+3| charge.
-
Elements in Group 14 (IV A) can lose four electrons and obtain a |+4| charge or gain four electrons and obtain a |-4| charge.
-
Elements in Group 15 (V A), tend to gain three electrons and obtain a |-3| charge.
-
Elements in Group 16 (VI A), tend to gain two electrons and obtain a |-2| charge.
-
Halogens, which are elements in Group 17 (VII A), tend to gain one electron and obtain a |-1| charge.
Reminder: Noble gases, which are the elements in Group 18 (VIII A), do not tend to lose or gain electrons. They do not form ions.
A polyatomic ion is an ion made up of a group of atoms bonded together.
Here are the chemical formulas of some polyatomic ions and their names.
|
Chemical formula |
|\text{CH}_{3}\text{COO}^{-}| |
|\text{NH}_{4}{^{+}}| |
|\text{HCO}_{3}{^{-}}| |
|\text{CO}_{3}{^{2-}}| |
|\text{ClO}_{3}{^{-}}| |
|\text{CrO}_{4}{^{2-}}| |
|\text{H}_{3}\text{O}^{+}| |
|\text{OH}^{-}| |
|\text{NO}_{3}{^{-}}| |
|\text{NO}_{2}{^{-}}| |
|\text{PO}_{4}{^{3-}}| |
|\text{SO}_{4}{^{2-}}| |
|\text{SO}_{3}{^{2-}}| |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Name |
Acetate |
Ammonium |
Bicarbonate |
Carbonate |
Chlorate |
Chromate |
Hydronium |
Hydroxide |
Nitrate |
Nitrite |
Phosphate |
Sulphate |
Sulphite |