The concentration of a solution is the ratio between the amount of dissolved solute and the amount of solution.
The amount of solvent should not be confused with the amount of solution.
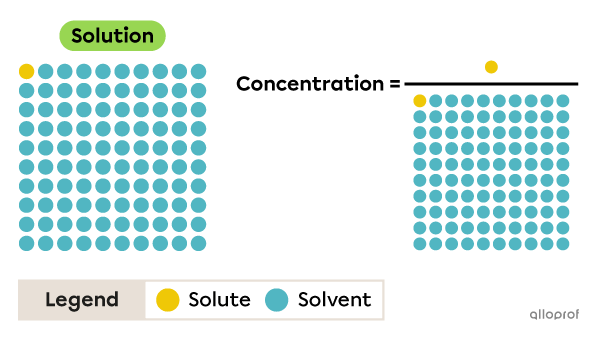
For example, the concentration of the solution shown in this image is represented by one part solute in 100 parts solution, and not one part solute in 99 parts solvent.
The concentration of a solution is influenced by different factors and can be expressed in different units.
Technically, chocolate milk is not a solution. It's not a homogeneous mixture, but rather a colloid. That said, the concept of concentration is easier to understand with delicious chocolate milk!
Concentration can be calculated using this general formula.
||C = \dfrac{Q_{solute}}{Q_{solution}}||
where
|C:| concentration
|Q_{solute}:| amount of solute
|Q_{solution}:| amount of solution
This formula shows that changing the amount of solute or the amount of solution affects the concentration of a solution. Usually, it is the amount of solvent that is changed to significantly alter the amount of solution.
The effects of various factors on concentration are summarized in the following table.
| Change made in the solution | Effect on concentration | Example |
|---|---|---|
| |\color{#7CCA51}{\nearrow}| Increase in the amount of solvent | |\color{#EC0000}{\searrow}| Concentration decreases | Diluting a solution. |
| |\color{#EC0000}{\searrow}| Decrease in the amount of solvent | |\color{#7CCA51}{\nearrow}| Concentration increases | Evaporating the solvent from a solution. |
| |\color{#7CCA51}{\nearrow}| Increase in the amount of solute | |\color{#7CCA51}{\nearrow}| Concentration increases | Dissolving more solute in a solution. |
| |\color{#EC0000}{\searrow}| Decrease in the amount of solute | |\color{#EC0000}{\searrow}| Concentration decreases | Evaporating a volatile solute (which evaporates easily) in a solution. |
There are several ways to express concentration, each one measured in different units. These include:
-
Concentration in g/L |(\text{g/L})| — Secondary 3
-
Percent concentration |(\text{%})| — Secondary 3
-
Parts per million concentration |(\text{ppm})| — Secondary 4 — ST
-
Molar concentration or molarity |(\text{mol/L})| — Secondary 4 — EST
There are other types of concentration.
For example, mole fraction is the ratio of the number of moles of solute to the total number of moles of solution. There is no unit for mole fraction.
Molality (not to be confused with molarity) is a type of concentration that represents the number of moles of solute divided by the solvent’s mass. This is one of the few units used to calculate concentration without taking the total amount of solution into account. Molality is expressed in |\text{mol/kg}| and is mainly used in industrial applications where it is easier to measure kilograms of solvent than to measure the total volume of a large amount of solution.