Electrical conductivity is the ability of a substance to flow through an electric current.
Electrical conductivity is measured qualitatively using an electrical conductivity detector (ECD) with indicator light. This type of ECD does not provide a numerical value for the degree of conductivity of a substance. By observing the indicator light on the ECD, it can be determined whether or not current is flowing through the substance.
When the ECD indicator light turns on, the substance conducts electricity.
When the ECD indicator light does not light up, the substance does not conduct electricity.
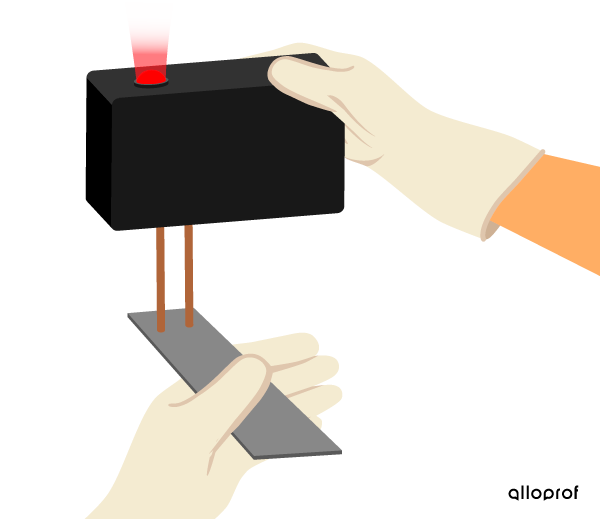
The aluminum rod conducts electricity.
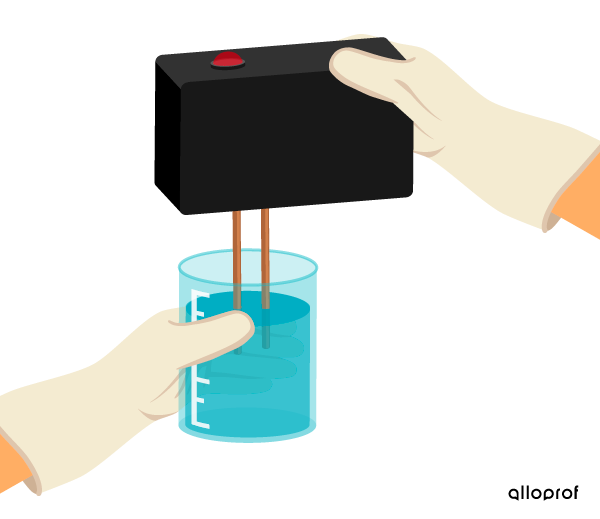
Pure water does not conduct electricity.
Materials that allow an electric current to flow are called conductors. Substances that do not allow an electric current to flow are referred to as insulators. On an atomic scale, the attraction of electrons to the nucleus of atoms is what differentiates conductors from insulators.
The electrons of a conductor are weakly attracted to the atom’s nucleus. This allows the free movement of electrons, and thus the flow of electric current. Most metals are excellent electrical conductors. Electrically conductive materials are used to perform the conduction function in electrical circuits.
Silver, copper, and gold are metals with high electrical conductivity.

demarcomedia, Shutterstock.com
raigvi, Shutterstock.com
The electrons of an insulator are strongly attracted by the atom’s nucleus. It is, therefore, difficult for electrons to move from one atom to another. As the flow of an electric current depends on the movement of electrons, the current does not flow through an insulating material.
Plastics, wood, and ceramics are electrical insulators. Insulating materials are used to perform the insulation function in electrical circuits.
Rubber and glass are electrical insulators.
asadykov, Shutterstock.com

Nail Bikbaev, Shutterstock.com
The electrical conductivity of conductive materials varies according to a number of factors.
The electrical conductivity depends on the attraction of electrons to the nucleus of the material’s atoms. When this attraction is weak, the electrical conductivity is high. The following table describes the conductivity of some conductive metals:
| Metal | Electrical conductivity (S/m) |
|---|---|
| Silver (Ag) | 6.30 × 107 |
| Copper (Cu) | 5.96 × 107 |
| Gold (Au) | 4.10 × 107 |
| Aluminum (Al) | 3.50 × 107 |
| Iron (Fe) | 1.00 x 107 |
| Mild steel (iron and carbon alloy) | 6.99 × 106 |
Electrical conductivity is measured in siemens per metre (S/m). In the previous table, we notice that the best conductors are silver, copper, and gold.
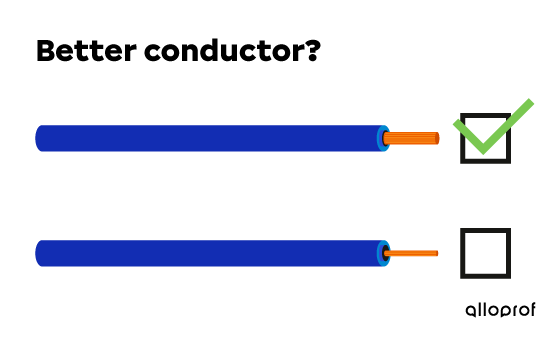
A wire with a larger diameter contains more atoms. This means that the flow rate of moving electrons in the wire is higher. A wire that allows electrons to move better, therefore, has better electrical conductivity.
For these two copper conductor wires of equal length, the thicker wire has the better electrical conductivity.
For these two copper conducting wires of equal length, the thicker wire has the better electrical conductivity.
The movement of electrons within a conducting wire leads to collisions between the other electrons and the insulating walls of the wire, which interferes with the passage of current. The shorter the wire, the less likely the electrons are to collide, making it easier for the current to flow.
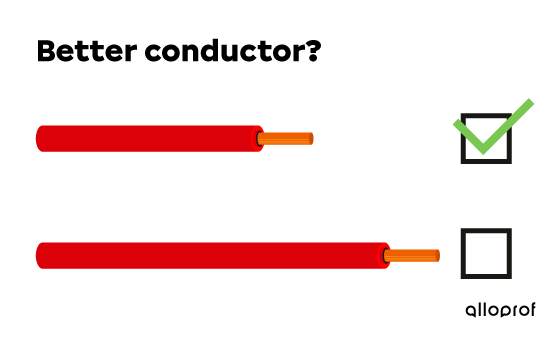
Thus, as the length of a conductor increases, the electrical conductivity decreases.
For these two copper conducting wires of equal diameter, the shorter wire has the best electrical conductivity.
The increase in the temperature of a conductor causes the agitation of its particles. This agitation adds to the disordered movement of electrons and increases the collisions in the wire. More collisions make it harder for electrons to move, which decreases conductivity.
Thus, when the temperature of a conductor increases, the electrical conductivity decreases.
A computer server room is designed to keep the air as cool as possible.
Ventilated shelves, air conditioning systems, and even coolants are used in such facilities.
Coolness allows the electrical circuits to maintain a better conductivity, thus better efficiency.

sdecoret, Shutterstock.com
Superconductivity is a phenomenon characterized by the absence of electrical resistance within a material. These superconductors have infinite electrical conductivity, which allows current to flow without any loss of energy. This phenomenon makes it possible to generate magnetic fields of very high intensity.
To achieve superconductivity, there must be extremely cold conditions, very close to absolute zero (-273.15 °C). Under these conditions, the particles are practically immobile, leaving the field open to the movement of electrons.
The ultra-fast movement of magnetic levitation (Maglev) trains is due to superconductivity. The Shanghai high-speed train runs from the city centre to the airport (30 km) in 7 minutes and 20 seconds.

cyo bo, Shutterstock.com
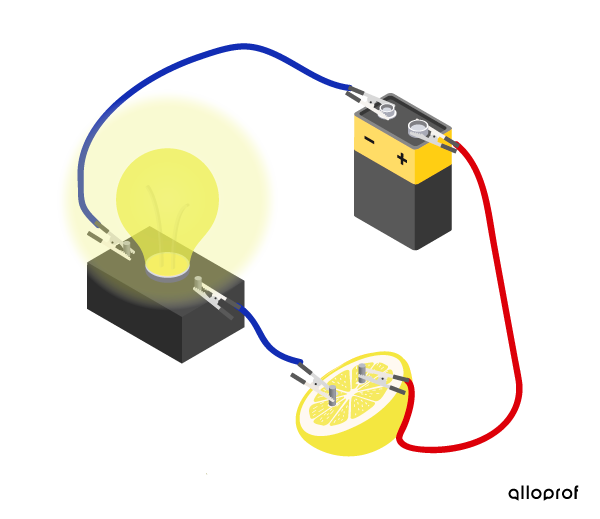
It's not just materials that can conduct an electric current. Electrolyte solutions, such as acid, base, or salt solutions conduct electric current, because they contain mobile ions. Both ions and electrons have an electrical charge. Their displacement allows the passage of an electric current. The electrical conductivity of a solution depends on its ion concentration.
Lemon juice (an acidic solution) contains enough mobile ions to allow current to flow from the battery to the bulb. The bulb lights up.