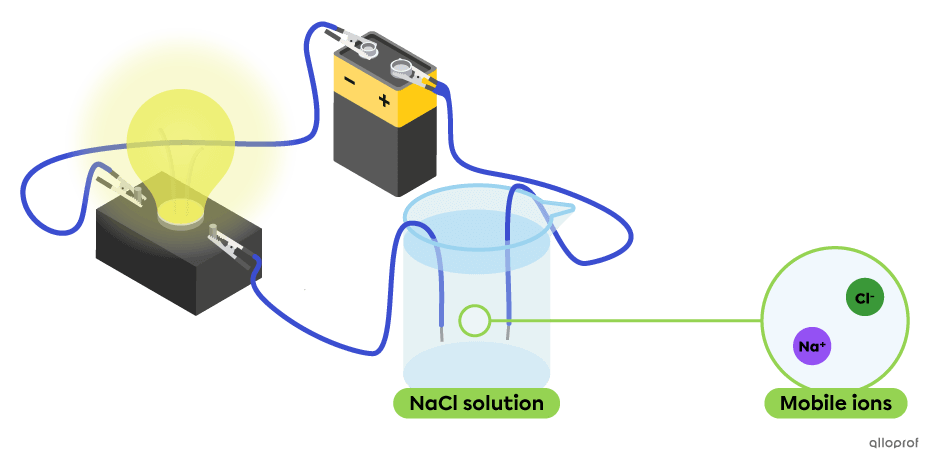
An aqueous solution allows an electric current to pass through it when it contains mobile ions. These ions are obtained from the dissociation of an electrolyte in water. The more mobile ions there are, the more conductive the solution.
In the following diagram, the aqueous solution of sodium chloride |(\text{NaCl}),| an electrolyte, contains mobile ions |\text{Na}^{+}| and |\text{Cl}^{-}.| This solution conducts an electric current, as evidenced by the light emitted by the light bulb.
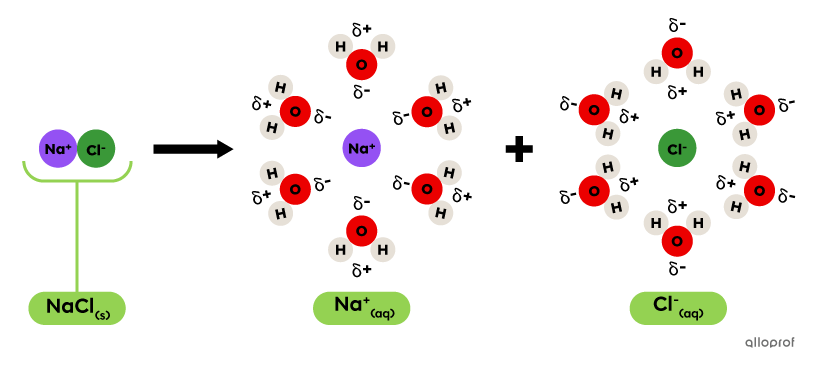
In a water molecule |(\text{H}_2\text{O}),| oxygen |(\text{O})| has a stronger pull on the electrons of the |\text{H-O}| bond because oxygen has higher electronegativity than hydrogen. A partial negative charge forms around oxygen and a partial positive charge forms around hydrogen. These charges are indicated by |\delta^-| and |\delta^+| symbols.
Because of the partial charges of water molecules, they are attracted to the particles of an electrolyte in a specific orientation. As a result, the molecules of water surround the ions and dissociation occurs.
The following diagram shows that:
-
The |\text{Na}^+| ion is surrounded by oxygen atoms |(\text{O})| of the water molecules with the |\delta^-| symbol. The partially negative charge of each water molecule is attracted to the positive charge of the |\text{Na}^+| ion.
-
The |\text{Cl}^-| ion is surrounded by hydrogen atoms |(\text{H})| of the water molecules with the |\delta^+| symbol. The partially positive charge of each water molecule is attracted to the negative charge of the |\text{Cl}^-| ion.
The dissociation of an electrolyte can be represented by a chemical equation where:
-
The electrolyte is the reactant.
-
The cation and the anion are the products and are found in the aqueous phase (aq).
-
The cation and anion carry their respective charges.
-
The sum of charges of the cation and anion must be |0| (zero).
Water |(\text{H}_2\text{O})| is not usually represented in the chemical equation. It is the aqueous state (aq) of the products that indicates that they are dissolved in water.
Here is the chemical equation for the electrolytic dissociation of sodium chloride |(\text{NaCl}).|
||\begin{align}\text{NaCl}_{\text{(s)}}\ \rightarrow \ \text{Na}^{+}{_{\text{(aq)}}}\ +\ \text{Cl}^{-}{_{\text{(aq)}}}\end{align}||
-
|\text{NaCl}_{\text{(s)}}| is the electrolyte in solid state.
-
|\text{Na}{^+}{_{\text{(aq)}}}| is the cation in aqueous state with the charge |+1|.
-
|\text{Cl}{^-}{_{\text{(aq)}}}| is the anion in aqueous state with the charge |-1|.
-
The sum of charges is |+1+(-1)=0.|
The following procedure can be used to write the chemical equation for electrolytic dissociation.
-
Write the chemical formula of the electrolyte with its state as a subscript |\text{(s)}| on the reactant side, then an arrow to the right.
-
Separate the electrolyte into its cation and its anion and write them on the product side.
-
Using the periodic table, determine the respective charges of the cation and the anion and write them as superscripts.
-
Indicate the aqueous phase |\text{(aq)}| of the ions as a subscript.
-
If the chemical formula of the electrolyte includes subscripts, they become the coefficients of the ions.
-
Calculate the sum of charges to confirm that it is |0.|
Here is the chemical equation for the electrolytic dissociation of solid magnesium chloride |(\text{MgCl}{_2}{_{\text{(s)}}}).|
-
Write the chemical formula |\text{MgCl}{_2}| and the solid state subscript on the reactant side, then an arrow to the right.
|\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow| -
Separate |\text{MgCl}{_2}| into the |\text{Mg}| cation and |\text{Cl}| anion and write the ions on the product side.
|\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Mg}\ +\ \text{Cl}| -
Using the periodic table, determine that the |\text{Mg}| ion has a charge of |+2| and the |\text{Cl}| ion has a charge of |-1.| Write them as superscripts.
|\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Mg}^{2+}\ +\ \text{Cl}^{-}| -
Indicate the aqueous phase of the ions.
|\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Mg}^{2+}{_{\text{(aq)}}}\ +\ \text{Cl}^{-}{_{\text{(aq)}}}| -
The subscript |2| in |\text{Cl}_2| becomes the coefficient |2| in front of the |\text{Cl}^-| ion.
|\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Mg}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{Cl}^{-}{_{\text{(aq)}}}| -
Calculate the sum of charges to confirm that it is |0.|
|\begin{alignat}{1}&\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow \ &\text{Mg}^{2+}{_{\text{(aq)}}}&\ +\ &2\ \text{Cl}^{-}{_{\text{(aq)}}}\\&&+2&\ +\ &2\times(-1)&\\&&+2&\ +\ &-2&=0\end{alignat}|
The chemical equation for the electrolytic dissociation of |\text{MgCl}{_2}{_{\text{(s)}}}| is |\text{Mg}\text{Cl}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Mg}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{Cl}^{-}{_{\text{(aq)}}}.|
Determine the chemical equation for the electrolytic dissociation of solid calcium nitride |(\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}).|
-
Write the chemical formula |\text{Ca}_{3}\text{N}{_{2}}| and the solid state subscript on the reactant side, then an arrow to the right.
|\begin{align}\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow\end{align}| -
Separate |\text{Ca}_{3}\text{N}{_{2}}| into the |\text{Ca}| cation and |\text{N}| anion and write the ions on the product side.
|\begin{align}\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}\ +\ \text{N}\end{align}| -
Using the periodic table, determine that the |\text{Ca}| ion has a charge of |+2| and the |\text{N}| ion has a charge of |-3.| Write them as superscripts.
|\begin{align}\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}^{2+}\ +\ \text{N}^{3-}\end{align}| -
Indicate the aqueous phase of the ions.
|\begin{align}\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}^{2+}{_{\text{(aq)}}}\ +\ \text{N}^{3-}{_{\text{(aq)}}}\end{align}| -
The subscript |3| in |\text{Ca}_{3}| becomes the coefficient |3| in front of the |\text{Ca}^{2+}| ion. The subscript |2| in |\text{N}_{2}| becomes the coefficient |2| in front of the |\text{N}^{3-}| ion.
|\begin{align}\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow \ 3\ \text{Ca}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{N}^{3-}{_{\text{(aq)}}}\end{align}| -
Calculate the sum of charges to confirm that it is |0.|
|\begin{alignat}{1}&\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow \ &3\ \text{Ca}^{2+}{_{\text{(aq)}}}&\ +\ &2\ \text{N}^{3-}{_{\text{(aq)}}}\\&&3\times(+2)&\ +\ &2\times(-3)&\\&&+6&\ +\ &-6&=0\end{alignat}|
The chemical equation for the electrolytic dissociation of |\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}| is |\text{Ca}_{3}\text{N}{_{2}}{_{\text{(s)}}}\ \rightarrow \ 3\ \text{Ca}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{N}^{3-}{_{\text{(aq)}}}.|
When an electrolyte contains one or several polyatomic ions, the same approach is used to write the dissociation equation. However, it is necessary to recognize the groups of atoms that form polyatomic ions and to know their charge. This information can usually be found in a table such as this one.
|
Chemical formula |
|\text{CH}_{3}\text{COO}^{-}| |
|\text{NH}_{4}{^{+}}| |
|\text{HCO}_{3}{^{-}}| |
|\text{CO}_{3}{^{2-}}| |
|\text{ClO}_{3}{^{-}}| |
|\text{CrO}_{4}{^{2-}}| |
|\text{H}_{3}\text{O}^{+}| |
|\text{OH}^{-}| |
|\text{NO}_{3}{^{-}}| |
|\text{NO}_{2}{^{-}}| |
|\text{PO}_{4}{^{3-}}| |
|\text{SO}_{4}{^{2-}}| |
|\text{SO}_{3}{^{2-}}| |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Name |
Acetate |
Ammonium |
Bicarbonate |
Carbonate |
Chlorate |
Chromate |
Hydronium |
Hydroxide |
Nitrate |
Nitrite |
Phosphate |
Sulphate |
Sulphite |
Determine the chemical equation for the electrolytic dissociation of solid sodium nitrate |(\text{NaNO}_{3}{_{\text{(s)}}}).|
-
Write the chemical formula |\text{Na(NO}_{3})| and the solid state subscript on the reactant side, then an arrow to the right.
|\text{NaNO}_{3}{_{\text{(s)}}}\ \rightarrow| -
Separate |\text{Na(NO}_{3})| into the |\text{Na}| cation and |\text{NO}{_{3}}| anion and write the ions on the product side.
|\text{NaNO}_{3}{_{\text{(s)}}}\ \rightarrow \ \text{Na}\ +\ \text{NO}{_{3}}| -
Using the periodic table and the polyatomic ion table, determine that the |\text{Na}| ion has a charge of |+1| and the |\text{NO}{_{3}}| ion has a charge of |-1.| Write them as superscripts.
|\text{NaNO}_{3}{_{\text{(s)}}}\ \rightarrow \ \text{Na}^{+}\ +\ \text{NO}{_{3}}^{-}| -
Indicate the aqueous phase of the ions.
|\text{NaNO}_{3}{_{\text{(s)}}}\ \rightarrow \ \text{Na}^{+}{_{\text{(aq)}}}\ +\ \text{NO}{_{3}}^{-}{_{\text{(aq)}}}| -
In this example, there are no subscripts in the electrolyte formula that become ion coefficients. The |3| in |\text{NO}{_{3}}| is part of the polyatomic ion.
-
Calculate the sum of charges to confirm that it is |0.|
|\begin{alignat}{1}&\text{NaNO}_{3}{_{\text{(s)}}}\ \rightarrow \ &\text{Na}^{+}{_{\text{(aq)}}}&\ +\ & \text{NO}{_{3}}^{-}{_{\text{(aq)}}}\\&&+1&\ +\ &-1&=0\end{alignat}|
The chemical equation for the electrolytic dissociation of |\text{Na(NO}_{3}){_{\text{(s)}}}| is |\text{NaNO}_{3}{_{\text{(s)}}}\ \rightarrow \ \text{Na}^{+}{_{\text{(aq)}}}\ +\ \text{NO}{_{3}}^{-}{_{\text{(aq)}}}.|
Determine the chemical equation for the electrolytic dissociation of solid calcium bicarbonate |(\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}).|
-
Write the chemical formula |\text{Ca(HCO}_{3}){_{2}}| and the solid state subscript on the reactant side, then an arrow to the right.
|\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow| -
Separate |\text{Ca(HCO}_{3}){_{2}}| into the |\text{Ca}| cation and |\text{HCO}{_{3}}| anion and write the ions on the product side.
|\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}\ +\ \text{HCO}{_{3}}| -
Using the periodic table and the polyatomic ion table, determine that the |\text{Ca}| ion has a charge of |+2| and the |\text{HCO}{_{3}}| ion has a charge of |-1.| Write them as superscripts.
|\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}^{2+}\ +\ \text{HCO}{_{3}}^{-}| -
Indicate the aqueous phase of the ions.
|\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}^{2+}{_{\text{(aq)}}}\ +\ \text{HCO}{_{3}}^{-}{_{\text{(aq)}}}| -
The subscript |2| in |\text{(HCO}_{3}){_{2}}| becomes the coefficient |2| in front of the |\text{HCO}{_{3}}^{-}| ion.
|\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{HCO}{_{3}}^{-}{_{\text{(aq)}}}| -
Calculate the sum of charges to confirm that it is |0.|
|\begin{alignat}{1}&\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow \ &\text{Ca}^{2+}{_{\text{(aq)}}}&\ +\ &2\ \text{HCO}{_{3}}^{-}{_{\text{(aq)}}}\\&&+2&\ +\ &2\times(-1)&\\&&+2&\ +\ &-2&=0\end{alignat}|
The chemical equation for the electrolytic dissociation of |\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}| is |\text{Ca(HCO}_{3}){_{2}}{_{\text{(s)}}}\ \rightarrow \ \text{Ca}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{HCO}{_{3}}^{-}{_{\text{(aq)}}}.|