A period is a horizontal row in the periodic table of elements.
The seven periods are numbered from top to bottom. Generally, we find the period number on the left side of the periodic table. In the following image, the periods are identified with different colours.
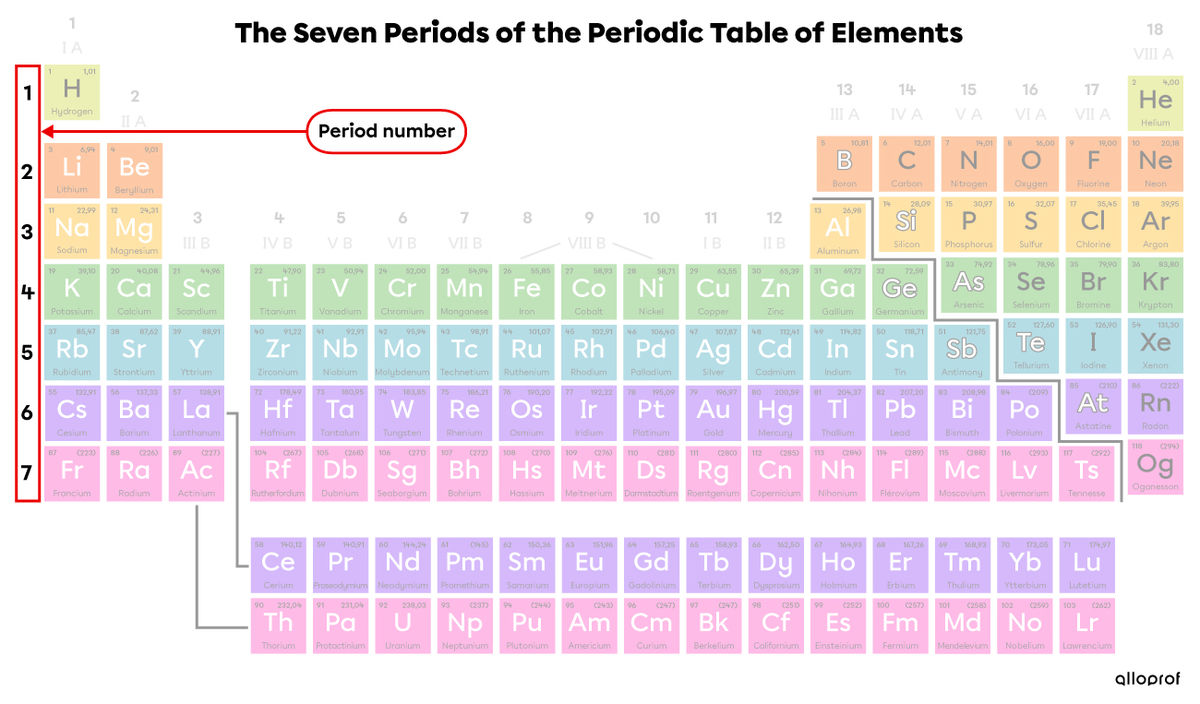
Lanthanides (elements with atomic numbers 58 to 71) belong to period 6.
Actinides (elements with atomic numbers 90 to 103) belong to period 7.
Pour valider ta compréhension à propos du tableau périodique de façon interactive, consulte la MiniRécup suivante :

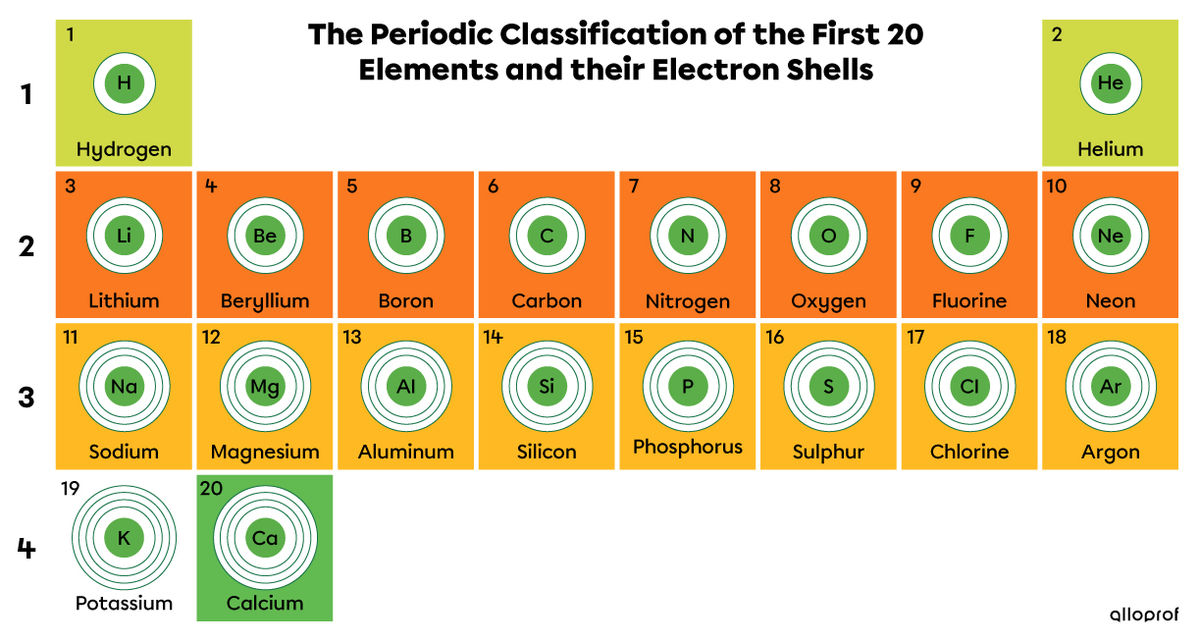
The classification of the elements into seven distinct periods makes it easy to determine the number of electron shells of the elements. This becomes very useful for representing atoms using the Rutherford-Bohr atomic model.
The number of electron shells of an element corresponds to its period number in the periodic table.
As shown in the image below, elements with a different number of electron shells do not belong to the same period. Conversely, elements that have an identical number of electron shells are found in the same period.
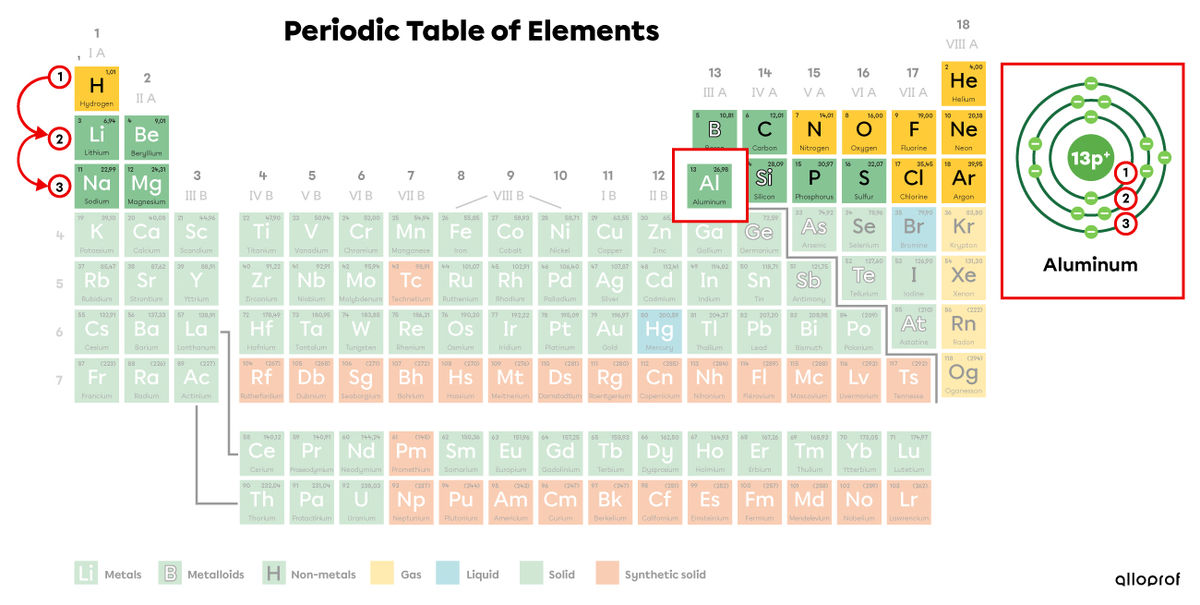
Aluminum (|\text{Al}|) is in the third period of the periodic table. Aluminum therefore has three electron shells, as represented by the Rutherford-Bohr atomic model.
Lithium (|\text{Li}|), beryllium (|\text{Be}|), boron (|\text{B}|), carbon (|\text{C}|), nitrogen (|\text{N}|), oxygen (|\text{O}|), fluorine (|\text{F}|), and neon (|\text{Ne}|) all belong to the second period of the periodic table.
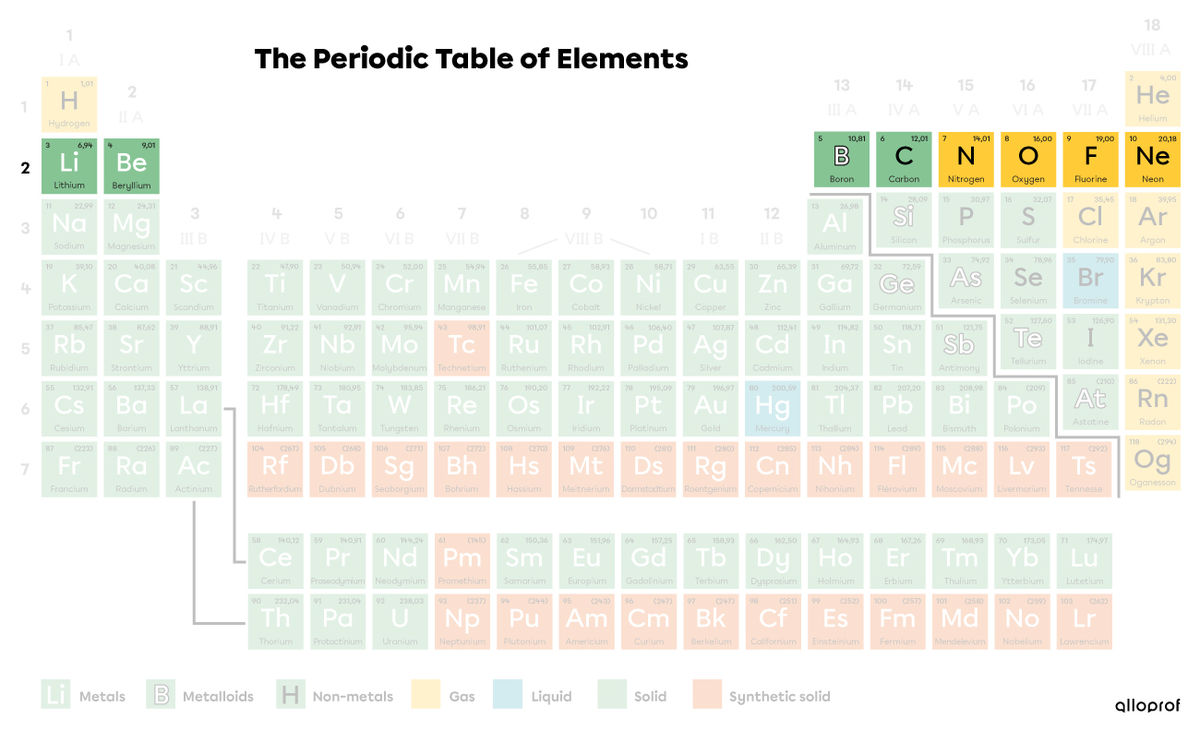
When analyzing their representations according to the Rutherford-Bohr model, notice how these elements all have two electron shells.
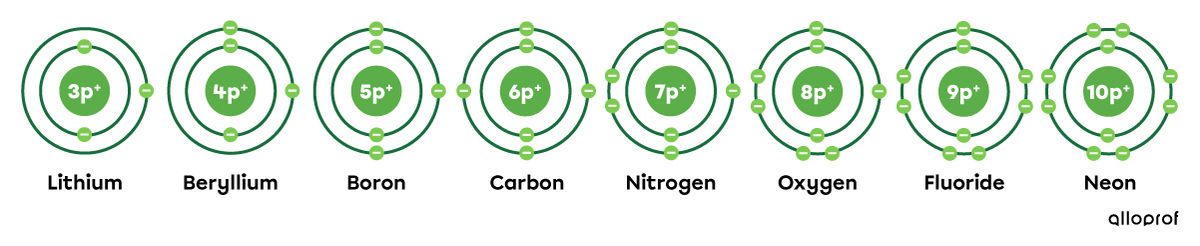
Here is the representation of selected elements in the periodic table above according to the Rutherford-Bohr atomic model.
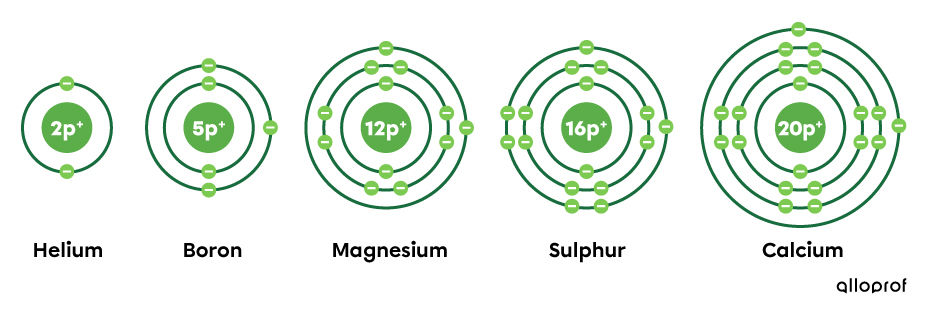
Looking at the period in which the selected elements are located and their representation, the following facts can be observed:
-
helium belongs to the first period, since it has one electron shell;
-
boron belongs to the second period, since it has two electron shells;
-
magnesium and sulphur are part of the third period, since they each have three electron shells;
-
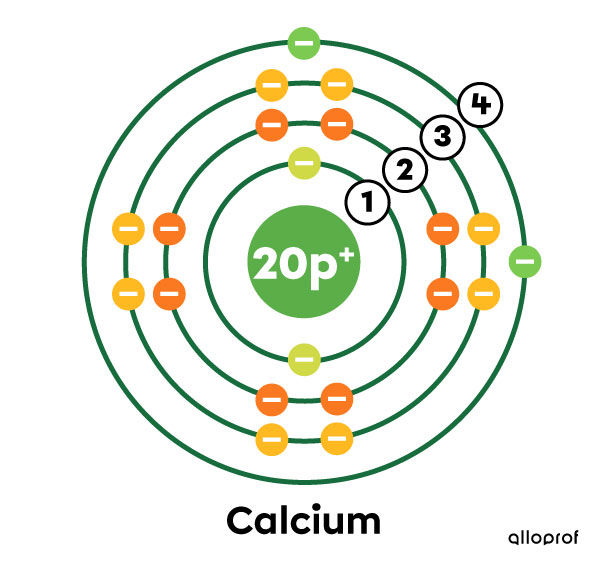
calcium belongs to the fourth period, since it has four electron shells.
The first period contains only hydrogen (|\text{H}|) and helium (|\text{He}|). As it contains only two elements, this period is easily forgotten when trying to locate it on the periodic table.
The electrons of an atom are located in its electron shells. The more electrons an atom has, the more electron shells it has to hold them. The way the electrons are distributed depends on the maximum capacity of each orbit.
The number of elements in a period of the periodic table corresponds to the maximum number of electrons that this electron shell can hold.
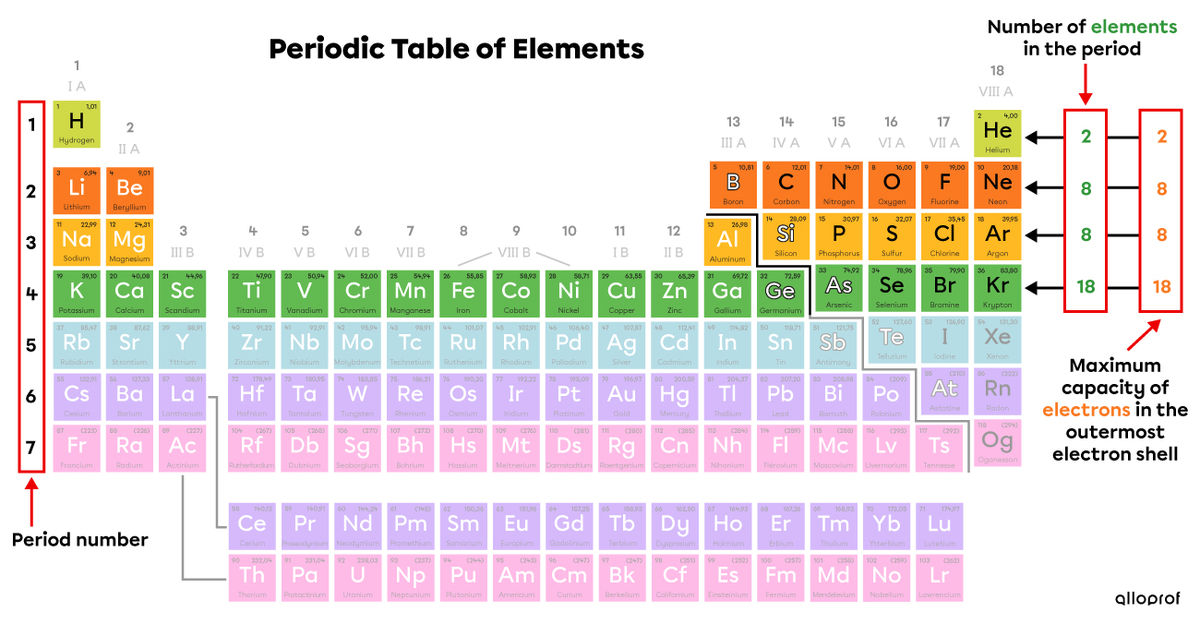
This trick is used to represent the distribution of electrons in the electron shells of elements 1 to 20. From atomic number 21 onwards, other theories are better suited to represent atoms.
The distribution of electrons in the electron shells is done in such a way as to saturate the shells closest to the nucleus first.
Calcium (|\text{Ca}|) has 20 electrons in total.
In the Rutherford-Bohr model, we observe that the first three electron shells are saturated with electrons (2, 8 and 8 electrons), while the fourth shell has only 2 electrons.
Pour valider ta compréhension à propos du tableau périodique de façon interactive, consulte la MiniRécup suivante :
