In aqueous solutions, an electrolyte dissociates into two ions, a positive ion called a cation and a negative ion called an anion. The ions obtained by the dissociation of the electrolyte can conduct an electric current in the aqueous solution.
Acids, bases and salts are electrolytes.
-
An electrolyte is a substance that allows the flow of an electric current when in an aqueous solution.
-
A nonelectrolyte is a substance that does not allow the flow of an electric current when in an aqueous solution.
In the following diagram, the aqueous solution of sodium chloride |(\text{NaCl}),| a salt, contains |\text{Na}^+| and |\text{Cl}^-| ions. This solution conducts an electric current, as evidenced by the light emitted by the light bulb. Sodium chloride |(\text{NaCl})| is an electrolyte.
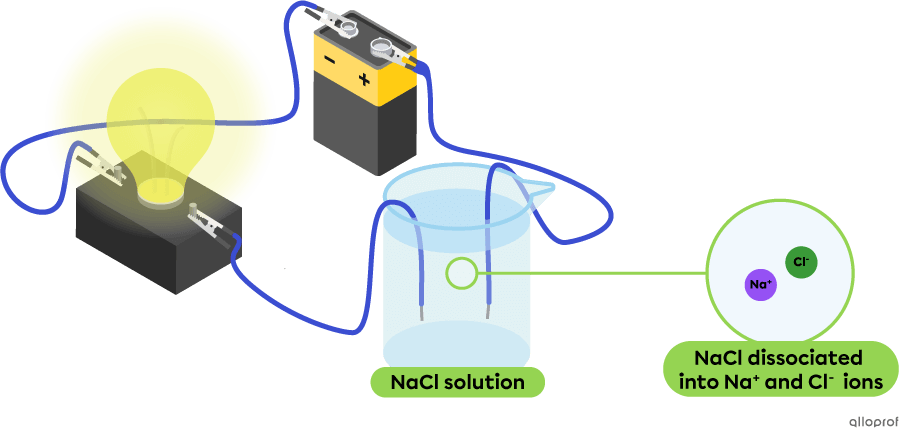
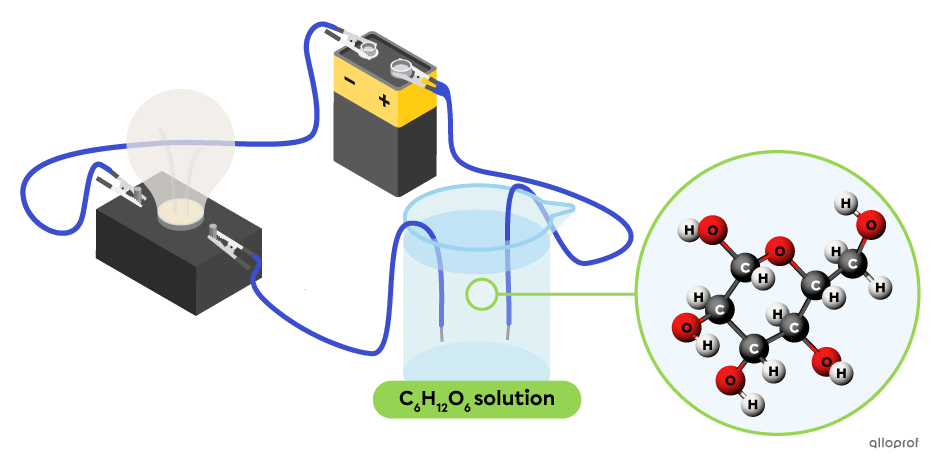
Glucose |(\text{C}_6\text{H}{_{12}}\text{O}_6),| oxygen |(\text{O}_2)| and carbon dioxide |(\text{CO}_2)| are examples of nonelectrolytes.
In the following diagram, the aqueous solution of glucose |(\text{C}_6\text{H}{_{12}}\text{O}_6)| does not contain ions. This solution does not conduct an electric current, as evidenced by the light bulb that does not emit light. Glucose |(\text{C}_6\text{H}{_{12}}\text{O}_6)| is a nonelectrolyte.
Here are a few general points about acids, bases and salts that are studied in secondary 4.
| Type of electrolyte | Acid | Base | Salt |
|---|---|---|---|
| Chemical formula | Hydrogen |(\text{H})| and a non-metal | A metal and a hydroxyl group |(\text{OH})| | A metal and a non-metal |
| Ions released in an aqueous solution | A proton |\text{H}^+| and an anion | A cation and a hydroxide |(\text{OH}^-)| | A cation and an anion |
| pH in an aqueous solution | Below |7| | Above |7| | Equal to |7| (neutral)* |
| Example | Hydrochloric acid |(\text{HCl})| | Sodium hydroxide |(\text{NaOH})| | Sodium chloride |(\text{NaCl})| |
Note: A salt dissolved in water does not always produce a neutral solution. For example, an aqueous solution of sodium fluoride |(\text{NaF})| has a basic pH.
- An acid is an electrolyte that allows the release of |\text{H}^+| ions in an aqueous solution.
- A |\text{H}^+| ion is called a proton.
The chemical formula of an acid usually begins with a hydrogen symbol |(\color{red}{\text{H}})| followed by a non-metal.
-
Hydrofluoric acid |(\color{red}{\text{H}}\text{F})|
-
Hydrochloric acid |(\color{red}{\text{H}}\text{Cl})|
-
Hydriodic acid |(\color{red}{\text{H}}\text{I})|
The fluorine |(\text{F}),| chlorine |(\text{Cl})| and iodine |(\text{I})| are non-metals.
The chemical formula of an acid can also begin with the hydrogen symbol |(\color{red}{\text{H}})| followed by a group of atoms.
-
Nitric acid |(\color{red}{\text{H}}\text{NO}_3)|
-
Sulfuric acid |(\color{red}{\text{H}_2}\text{SO}_4)|
The chemical formula of acetic acid |(\text{CH}{_3}\text{COO}\color{red}{\text{H}})| ends with the hydrogen symbol |(\color{red}{\text{H}}).|
In the chemical equation for the electrolytic dissociation of an acid, the hydrogen ion is in the aqueous phase |(\text{H}{^+}{_{\text{(aq)}}}).|
-
|\text{HF}_{\text{(g)}}\rightarrow\text{H}^{+}{_{\text{(aq)}}}+\text{F}^{-}{_{\text{(aq)}}}|
-
|\text{H}_2\text{SO}_{4}{_{\text{(l)}}}\rightarrow2\ \text{H}^{+}{_{\text{(aq)}}}\ +\ \text{SO}_{4}{^{2-}}{_\text{(aq)}}|
-
|\text{CH}_{3}\text{COOH}_{\text{(l)}}\rightarrow\text{H}^{+}{_{\text{(aq)}}}+\text{CH}_{3}\text{COO}^{-}{_{\text{(aq)}}}|
- A base is an electrolyte that allows the release of |\text{OH}^-| ions in an aqueous solution.
- A |\text{OH}^-| ion is called a hydroxide.
The chemical formula of a base usually begins with a metal followed by a hydroxyl group |(\color{blue}{\text{OH}}).|
-
Lithium hydroxide |(\text{Li}\color{blue}{\text{OH}})|
-
Sodium hydroxide |(\text{Na}\color{blue}{\text{OH}})|
-
Calcium hydroxide |(\text{Ca(}\color{blue}{\text{OH}})_2)|
Lithium |(\text{Li}),| sodium |(\text{Na})| and calcium |(\text{Ca})| are metals.
The chemical formula of ammonia |(\text{NH}_3)| does not end with |\text{OH}.| Ammonia reacts with water |(\text{H}{_2}\text{O})| according to the following equation:
||\text{NH}_{3}{_{\text{(g)}}}+\text{H}_2\text{O}{_\text{(l)}}\rightarrow\text{NH}{_4}^{+}{_{\text{(aq)}}}+\text{OH}^{-}{_{\text{(aq)}}}||
The reaction between |\text{NH}_3| and water releases |\text{OH}^-| ions, which means that |\text{NH}_3| is a base.
The chemical formula of a base can also begin with a group of atoms followed by a hydroxyl group |(\color{blue}{\text{OH}}).|
Ammonium hydroxide |(\text{NH}{_4}\color{blue}{\text{OH}})| is formed of a group of atoms |\text{NH}{_4}| and a hydroxyl group |\text{OH}.|
The chemical formula of the acetic acid |(\text{CH}{_3}\text{COOH})| ends with |\text{OH},| but it is not a base. Acetic acid dissociates in water according to the following equation: ||\text{CH}_{3}\text{COOH}_{\text{(l)}}\rightarrow\text{H}^{+}{_{\text{(aq)}}}+\text{CH}_{3}\text{COO}^{-}{_{\text{(aq)}}}||
The dissociation releases |\text{H}^+| ions, which means that |\text{CH}{_3}\text{COOH}| is an acid.
In the chemical equation for the electrolytic dissociation of a base, the hydroxide ion is in the aqueous phase |(\text{OH}{^-}{_{\text{(aq)}}}).|
- |\text{NaOH}_{\text{(s)}}\rightarrow\text{Na}^{+}{_{\text{(aq)}}}+\text{OH}^{-}{_{\text{(aq)}}}|
- |\text{Mg}\text{(OH)}_{2}{_{\text{(s)}}}\rightarrow\text{Mg}^{2+}{_{\text{(aq)}}}\ +\ 2\ \text{OH}{^{-}}{_\text{(aq)}}|
- |\text{NH}_4\text{OH}{_{\text{(aq)}}}\rightarrow\text{NH}{_4}^{+}{_{\text{(aq)}}}\ +\ \text{OH}{^{-}}{_\text{(aq)}}|
A salt is an electrolyte usually made up of a metal and a non-metal.
-
Lithium fluoride |(\text{LiF})|
-
Sodium chloride |(\text{NaCl})|
-
Calcium oxyde |(\text{CaO})|
Lithium |(\text{Li}),| sodium |(\text{Na})| and calcium |(\text{Ca})| are metals.
Fluorine |(\text{F}),| chlorine |(\text{Cl})| and oxygen |(\text{O})| are non-metals.
The chemical formula of a salt can also contain groups of atoms.
-
Sodium nitrate |(\text{NaNO}_3)| contains sodium |(\text{Na}),| a metal and a group of atoms |\text{NO}{_3}.|
-
Ammonium chloride |(\text{NH}{_4}\text{Cl})| contains a group of atoms |\text{NH}_4| and chlorine |(\text{Cl}),| a non-metal.
-
Ammonium nitrate |(\text{NH}{_4}\text{NO}{_3})| contains a group of atoms |\text{NH}_4| and another group of atoms |\text{NO}{_3}.|
In the chemical equation for the electrolytic dissociation of a neutral salt, the ions are usually other than the proton |(\text{H}{^+}{_{\text{(aq)}}})| and the hydroxide |(\text{OH}{^-}{_{\text{(aq)}}}).|
- |\text{NaCl}_{\text{(s)}}\rightarrow\text{Na}^{+}{_{\text{(aq)}}}+\text{Cl}^{-}{_{\text{(aq)}}}|
- |\text{CaBr}{_2}_{\text{(s)}}\rightarrow\text{Ca}^{2+}{_{\text{(aq)}}}+2\ \text{Br}^{-}{_{\text{(aq)}}}|
- |\text{K}\text{NO}{_3}_{\text{(s)}}\rightarrow\text{K}^{+}{_{\text{(aq)}}}+\text{NO}{_3}^{-}{_{\text{(aq)}}}|
The rate of dissociation varies from one electrolyte to another, which influences the electrical conductivity of an electrolytic solution. The higher the dissociation rate, the more easily the solution conducts current.
Electrolytes with a very high dissociation rate (close to 100%) are strong electrolytes. Electrolytes with a low dissociation rate are weak electrolytes.
-
A strong electrolyte is one that dissociates almost completely in water.
-
A weak electrolyte is one that partially dissociates in water.
In the following diagram, all the particles of sodium chloride |(\text{NaCl})| dissociate into |(\text{Na}^+)| and |(\text{Cl}^-)| ions. This solution conducts the electric current very well, as evidenced by the bright light emitted by the light bulb. Sodium chloride |(\text{NaCl})| is a strong electrolyte.
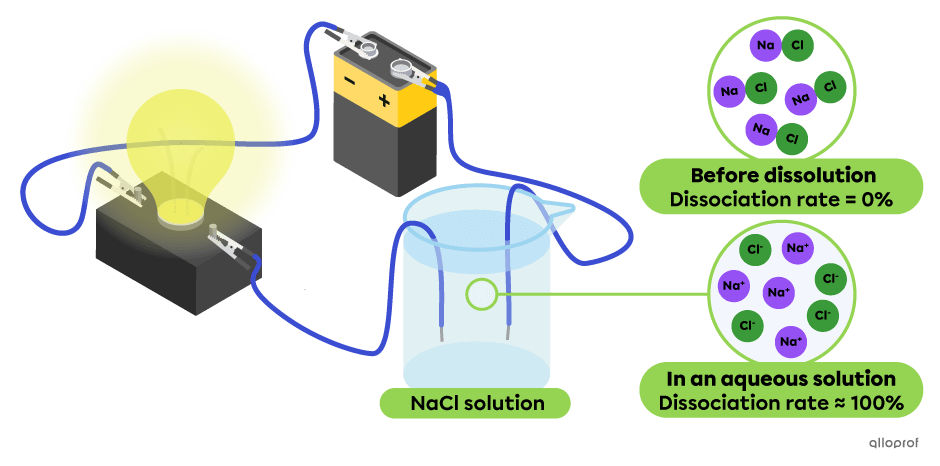
In the following diagram, all the particles of silver chloride |(\text{AgCl})| dissociate into |(\text{Ag}^+)| and |(\text{Cl}^-)| ions. This solution conducts the electric current but not well, as evidenced by the dim light emitted by the light bulb. Silver chloride |(\text{AgCl})| is a weak electrolyte.
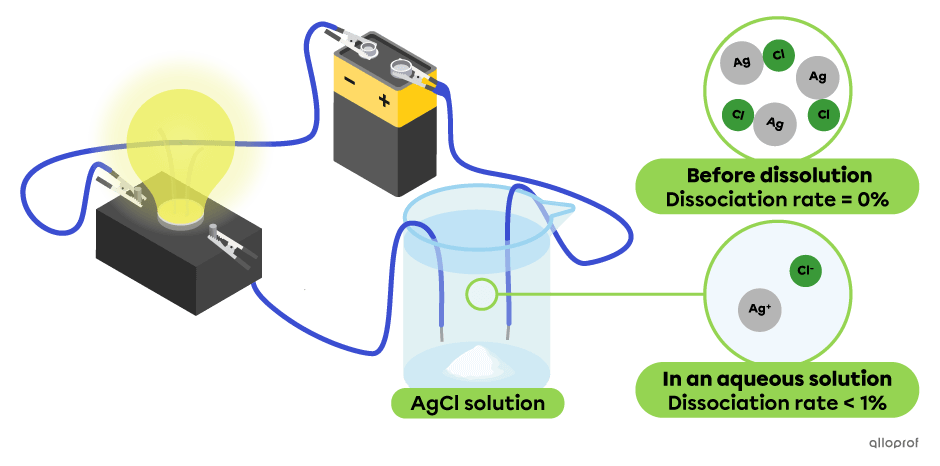
Note: The dissociation rate of silver chloride (AgCl) is calculated for a concentration of 0.1 mol/L.
The following table lists several strong electrolytes and their chemical formulas.
|
Type of electrolyte |
Name |
Chemical formula |
|---|---|---|
|
Hydrochloric acid |
|\text{HCl}| |
|
|
Sulphuric acid |
|\text{H}{_2}\text{SO}{_4}| |
|
|
Sodium hydroxide |
|\text{NaOH}| |
|
|
Sodium chloride |
|\text{NaCl}| |
|
|
Potassium chloride |
|\text{KCl}| |
The following table lists several weak electrolytes and their chemical formulas.
|
Type of electrolyte |
Name |
Chemical formula |
|---|---|---|
|
Hydrofluoric acid |
|\text{HF}| |
|
|
Nitrous acid |
|\text{HNO}_2| |
|
|
Chlorous acid |
|\text{HClO}_2| |
|
|
Acetic acid |
|\text{CH}{_3}\text{COOH}| |
|
|
Ammonia |
|\text{NH}_3| |
|
|
Silver chloride |
|\text{AgCl}| |
|
|
Aluminum chloride |
|\text{AlCl}_3| |
|
|
Lead chloride |
|\text{PbCl}_2| |