The rules of chemical notation are used to write the chemical formula (also called molecular formula) of a compound.
The following sections illustrate the notation rules that apply to binary compounds made of a metal and a nonmetal. A chemical bond between a metal and a nonmetal requires the formation of ions. Therefore, these sections focus specifically on binary ionic compounds.
To determine the chemical formula of a binary ionic compound, the charges carried by the ions that make up the compound have to be determined first.
The octet rule can be used to determine an ionic charge. It explains the element’s tendency to gain or lose electrons to form an ion.
The periodic table below shows the ionic charge(s) carried by an element in a binary ionic compound. This table applies to Elements 1 to 54.
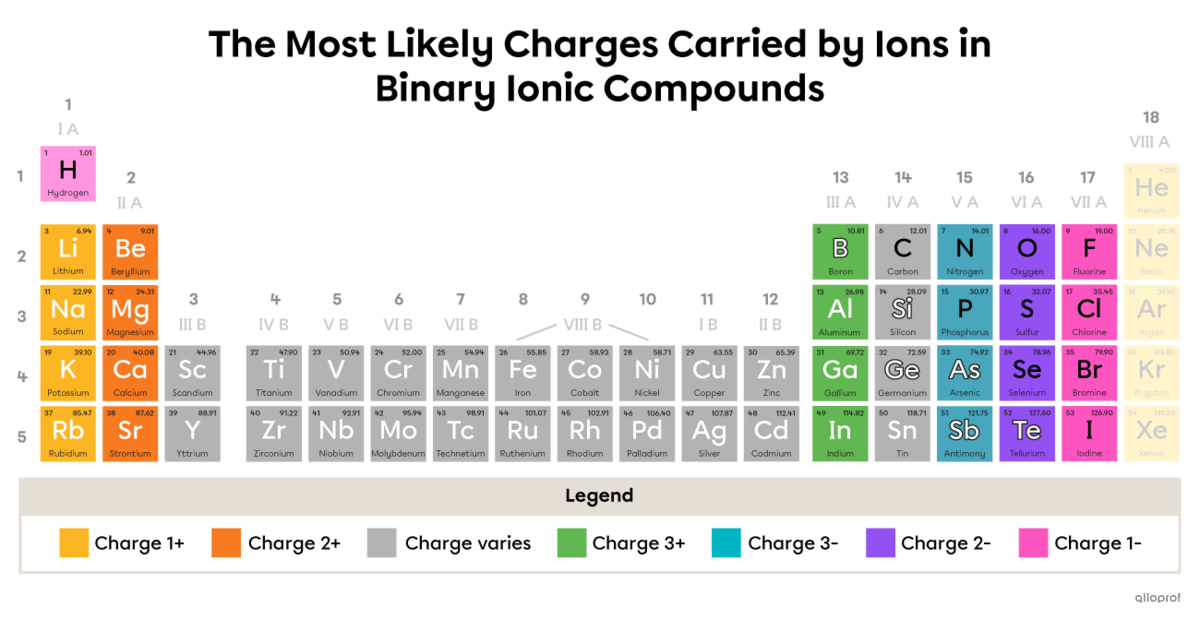
The charges indicated in the table represent the most likely charges carried by an ion in a binary ionic compound. Other charges are rare but possible.
In addition, the charges indicated in the table apply to binary ionic compounds. If a compound is ionic, but not binary, other ionic charges are possible. Ions are not involved in the formation of nonionic compounds.
In a binary ionic compound, what is the most likely charge of a magnesium ion?
Magnesium |(\text{Mg})| is the 12th element and belongs to Group II A. It has 2 valence electrons. To respect the octet rule, magnesium tends to lose the 2 electrons, becoming an ion with a |2+| charge: |\text{Mg}^{2+}.|
The periodic table in the previous section can be used to quickly determine the ionic charge of magnesium.

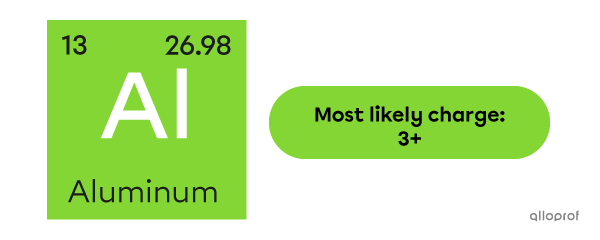
In a binary ionic compound, what is the most likely charge of an aluminum ion?
Aluminum |(\text{Al})| is the 13th element and belongs to Group III A. It has 3 valence electrons. To respect the octet rule, magnesium tends to lose the 3 electrons, becoming an ion with a |3+| charge: |\text{Al}^{3+}.|
The periodic table in the previous section can be used to quickly determine the ionic charge of aluminum.
In a binary ionic compound, what is the most likely charge of a copper ion?
Copper |(\text{Cu})| is the 29th element. The periodic table suggests that different charges are possible when the element becomes an ion. There is not enough information to answer the question.
In addition, the octet rule does not apply to transition metals (metals in Groups 3 to 12), so it cannot be used to determine the ionic charge of copper.

To determine the chemical formula of a binary ionic compound based on its elements, the following rules of chemical notation should be used.
-
Determine the ionic charges carried by the elements that make up the compound.
-
Determine the number of ions of each element required to create an electrically neutral compound. The sum of all ionic charges must be equal to |0.|
-
Write the chemical formula with the metal first and the nonmetal last.
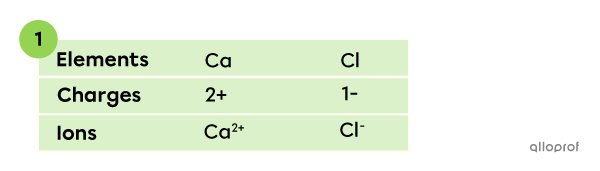
A compound is made of calcium |(\text{Ca})| and chlorine |(\text{Cl}).| What is the chemical formula of the compound?
-
In this binary ionic compound, calcium appears as a |\text{Ca}^{2+}| ion and chlorine as a |\text{Cl}^{-}| ion. The charges can be established based on the octet rule and the periodic table.
-
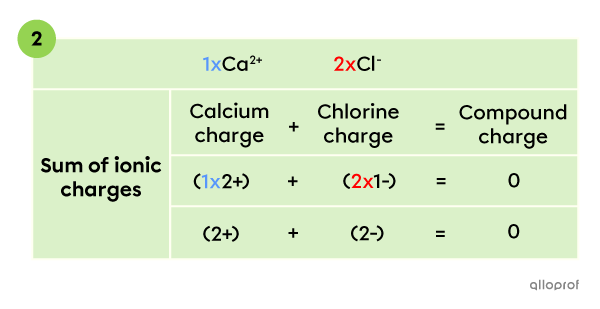
To create an electrically neutral compound, the sum of all ionic charges has to be |0.| Therefore, the compound contains 1 |\text{Ca}^{2+}| ion and 2 |\text{Cl}^{-}| ions, whose charges add up to zero.
-
Calcium |(\text{Ca})| is a metal, so it is written at the beginning of the chemical formula. Chlorine |(\text{Cl})| is a nonmetal, therefore, it is written at the end of the chemical formula. A subscript |2| indicates there are 2 chlorine atoms in the compound.
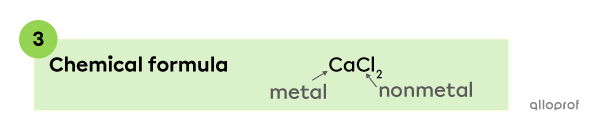
The chemical formula of a compound made of calcium |(\text{Ca})| and chlorine |(\text{Cl})| is |\text{CaCl}_{2}.|
Note: The ionic charge of the elements that make up |\text{CaCl}_2| are not included in the chemical formula. The answer is not |\text{Ca}^{2+} \text{Cl}^{-}_2,| but |\text{CaCl}_{2}.|
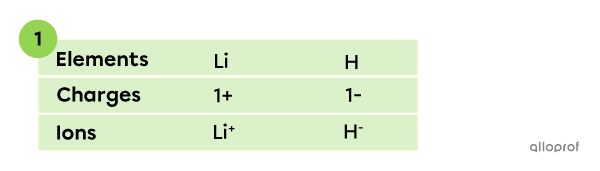
A compound is made of lithium |(\text{Li})| and hydrogen |(\text{H}).| What is the chemical formula of the compound?
-
In this binary ionic compound, lithium appears as a |\text{Li}^+| ion and hydrogen as a |\text{H}^-| ion. The charges can be established based on the octet rule and the periodic table.
-
To create an electrically neutral compound, the sum of all ionic charges has to be |0.| Therefore, the compound contains 1 |\text{Li}^+| ion and 1 |\text{H}^-| ion, whose charges add up to zero.
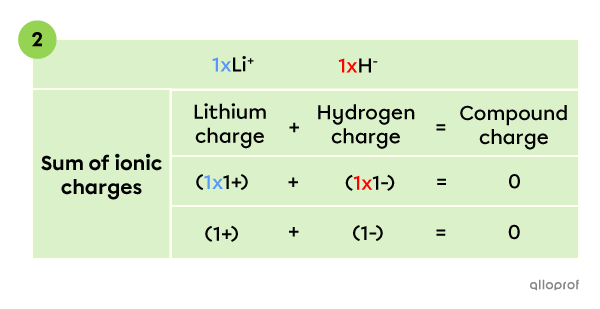
-
Lithium |(\text{Li})| is a metal, so it is written at the beginning of the chemical formula. Hydrogen |(\text{H})| is a nonmetal, therefore, it is written at the end of the chemical formula.
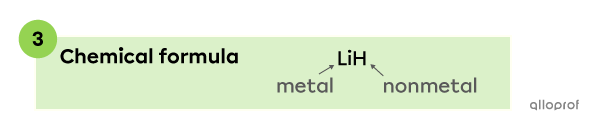
The chemical formula of a compound made of lithium |(\text{Li})| and hydrogen |(\text{H})| is |\text{LiH}.|
Note: The ionic charge of the elements that make up |\text{LiH}| are not included in the chemical formula. The answer is not |\text{Li}^{+} \text{H}^{-},| but |\text{LiH}.|
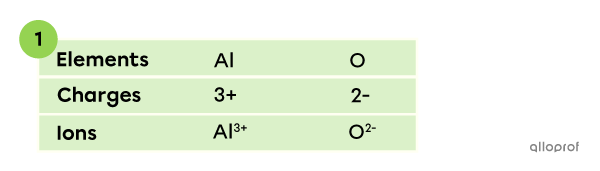
A compound is made of aluminum |(\text{Al})| and oxygen |(\text{O}).| What is the chemical formula of the compound?
-
In this binary ionic compound, aluminum appears as a |\text{AL}^{3+}| ion and oxygen as a |\text{O}^{2-}| ion. The charges can be established based on the octet rule and the periodic table.
-
To create an electrically neutral compound, the sum of all ionic charges has to be |0.| Therefore, the compound contains 2 |\text{Al}^{3+}| ions and 3 |\text{O}^{2-}| ions, whose charges add up to zero.
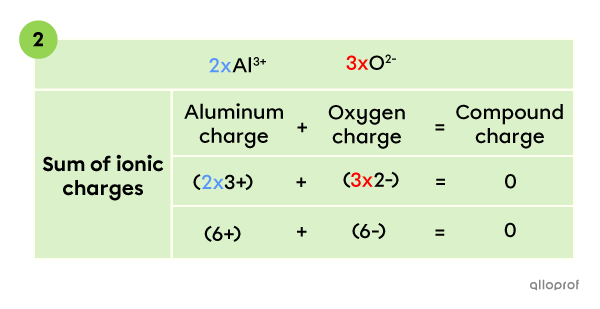
-
Aluminum |(\text{Al})| is a metal, so it is written at the beginning of the chemical formula. Oxygen |(\text{O})| is a nonmetal, therefore, it is written at the end of the chemical formula. Subscripts |2| and |3| indicate there are 2 aluminum atoms and 3 oxygen atoms in the compound.
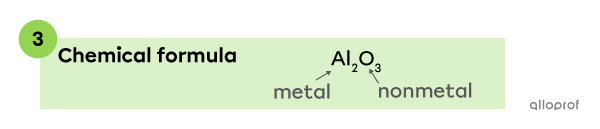
The chemical formula of a compound made of aluminum |(\text{Al})| and oxygen |(\text{O})| is |\text{Al}_2 \text{O}_{3}.|
Note: The ionic charge of the elements that make up |\text{Al}_2 \text{O}_{3}| are not included in the chemical formula. The answer is not |{\text{Al}^{3+}}_2 {\text{O}^{2-}}_{3},| but |\text{Al}_2 \text{O}_{3}.|