Nomenclature rules are used to name elements and compounds based on their chemical formulas.
The chemical composition of a substance named using clearly defined rules is easy to establish. For example, lithium chloride is a compound made up of lithium |(\text{Li})| and chlorine |(\text{Cl})|. More specifically, its chemical formula is |\text{LiCl}.|
Different chemical nomenclature rules apply depending on the type of substance.
Before chemistry became an established branch of science, various pure substances were discovered. To distinguish them, they were named after their place of origin (Epsom salt |(\text{MgSO}_{4})| was discovered in a town called Epsom), their mythological connections (ammonia |(\text{NH}_{3}),| after the Egyptian god Amun), or even their taste (lead sugar |(\text{Pb(CH}_3 \text{COO)}_{2})|).
Over time, countless substances were discovered, making it difficult to remember their names. Chemists decided to develop a universal language for naming substances logically, according to the atoms of which they are composed. The naming rules are known as chemical nomenclature.
Chemical nomenclature has existed since the 18th century. It evolved over time, leading to several nomenclature standards today. Therefore, the nomenclature rules may be different from one textbook to another.
The rules explained below were established before 2005 by the International Union of Pure and Applied Chemistry (IUPAC)[1]. Although IUPAC released an updated guide since, the nomenclature rules established before 2005 remain the most common ones in the field of chemistry.
While formal nomenclature rules apply to all substances, some of them have alternative common names that are used very frequently. For example, |\text{H}_2 \text{O}| is commonly referred to as water instead of dihydrogen monoxide.
An element is a substance made up of only one type of atom.
An element can be named using the following nomenclature rules.
-
If an element contains a single atom, it preserves the name indicated in the periodic table with the possible addition of the word atom.
-
If an element contains more than one atom, it preserves the name indicated in the periodic table with added terminology indicating that it is a molecule.
The number of atoms of a given element is indicated in the chemical formula using a subscript (|\text{O}_{\color{#7CCA51}{2}}, \text{O}_{\color{#7CCA51}{3}},| etc.).
The element |\text{O}| is made up of a single atom. It is referred to as oxygen, or an oxygen atom, as indicated in the periodic table.
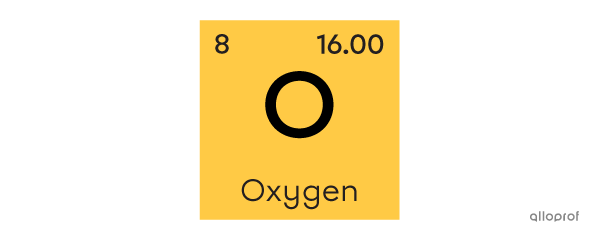
The element |\text{O}_2| is made up of 2 oxygen atoms. When 2 or more atoms of the same element are grouped together, a molecule is formed. |\text{O}_2| is referred to as molecular oxygen, or oxygen gas, because its most abundant state is gaseous.
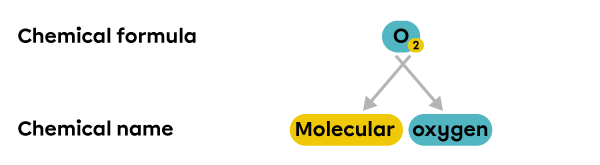
Nomenclature rules can also be used to determine the chemical formula based on the chemical name of the compound.
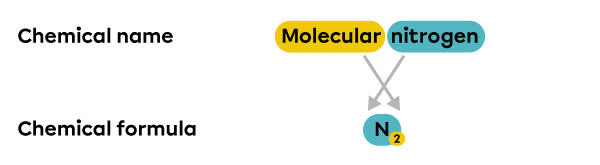
An element called molecular nitrogen refers to a group of nitrogen atoms, as opposed to a single nitrogen atom |(\text{N}).| Its chemical formula is |\text{N}_{2}.|
Note: The material that will help determine the number of atoms necessary to obtain the molecular form of an element (e.g., N2, N4, etc.) will be covered in Secondary 5 chemistry.
A covalent binary compound, such as |\text{P}_2 \text{O}_5|, is composed of two types of nonmetal atoms: |\text{P}| (phosphorus) and |\text{O}| (oxygen).
An ionic binary compound, such as |\text{NaCl}| is composed of a metal and a nonmetal atom: |\text{Na}| (sodium) and |\text{Cl}| (chlorine).
Covalent binary compounds, also referred to as binary molecular compounds, are made up of nonmetal atoms. They share electrons and form covalent bonds.
The periodic table can be used to identify nonmetals. The following nomenclature rules can be used to name binary covalent compounds.
-
Start by naming the atom that appears first in the chemical formula.
-
Use the element name in the periodic table.
-
If applicable, add a prefix (mono-, di-, tri-, etc.) to indicate the number of atoms of the element in the compound.
Note: The prefix mono- is often omitted.
-
-
Name the second atom.
-
Alter the element name, add the suffix -ide to the root.
-
If applicable, add a prefix (mono-, di-, tri-, etc.) to indicate the number of atoms of the element in the compound.
-
The number of atoms of an element is indicated in the chemical formula using a subscript (|\text{N}_{\color{#7CCA51}{2}} \text{O}_{\color{#7CCA51}{4}}, \text{Fe}_{\color{#7CCA51}{2}} \text{O}_{\color{#7CCA51}{3}},| etc.). The following table indicates the prefixes corresponding to the subscripts.
| Subscript | Prefix |
|---|---|
| 1 | Mono- |
| 2 | Di- |
| 3 | Tri- |
| 4 | Tetra- |
| 5 | Penta- |
| 6 | Hexa- |
| 7 | Hepta- |
| 8 | Octa- |
| 9 | Nona- |
| 10 | Deca- |
Note: The prefix mono- is only used when the same elements can combine in several different ways. For example, it is used to distinguish carbon monoxide |\text{CO}| from carbon dioxide |\text{CO}_2| or nitrogen monoxide |\text{NO}| from dinitrogen oxide |\text{N}_2 \text{O}.|
The following table indicates the names of some elements commonly found as the second element in a binary compound. Their elemental names are altered with the suffix -ide.
| Element name | Name used for the 2nd element in a covalent compound |
|---|---|
| Hydrogen | Hydride |
| Carbon | Carbide |
| Nitrogen | Nitride |
| Oxygen | Oxide |
| Phosphorus | Phosphide |
| Sulphur | Sulphide |
| Fluorine | Fluoride |
| Chlorine | Chloride |
| Iodine | Iodide |
In some instances, when the word oxide is preceded by a prefix, the last vowel of the prefix has to be removed, as shown in the following table.
| Prefix | Prefix + oxide | |
|---|---|---|
| Mono- | Monoxide | |
| Tetra- | Tetroxide | |
| Penta- | Pentoxide | |
Use the rules of nomenclature to name the compound with the following chemical formula: |\text{CO}_2.|
The covalent compound |\text{CO}_2| is made up of 1 carbon atom |\text{C}| and 2 oxygen atoms |\text{O}|.
-
Since the |\text{C}| atom appears first, it should be named first using its element name in the periodic table: carbon.
-
The element name of the second atom |\text{O}| has to be converted using the suffix -ide: it becomes oxide. A prefix di- is required because there are 2 |\text{O}| atoms.
The name of the compound with the chemical formula |\text{CO}_2| is carbon dioxide.
Le suffixe -ure indique que l’atome en question a une charge négative (et/ou un nombre d’oxydation négatif).
Par exemple, on prend le composé |\text{NaCl}.| Ce composé comprend les atomes |\text{Na}| et |\text{Cl},| qui se trouvent sous forme d’ions |\text{Na}^+| et |\text{Cl}^{-}.| Ainsi, |\text{Cl}^{-}| a une charge négative. Pour exprimer cela, |\text{Cl}^{-}| se nomme chlorure. Le composé |\text{NaCl}| se nomme donc chlorure de sodium.
Par contre, le gaz |\text{Cl}_2| comprend deux atomes de chlore neutres. Le gaz en question est donc nommé dichlore, et non dichlorure.
Use the nomenclature rules to name the compound with the following chemical formula: |\text{N}_2 \text{O}_4.|
The covalent compound |\text{N}_2 \text{O}_4| is made up of 2 nitrogen atoms |\text{N}| and 4 oxygen atoms |\text{O}|.
-
Since the |\text{N}| atom appears first in the chemical formula, it should be named first using its element name in the periodic table: nitrogen. A prefix di- is required because there are 2 |\text{N}| atoms.
-
The element name of the second atom |\text{O}| has to be converted using the suffix -ide: it becomes oxide. A prefix tetra- is required because there are 4 |\text{O}| atoms. Remember to remove the last vowel of the prefix.
The name of the compound with the chemical formula |\text{N}_2 \text{O}_4| is dinitrogen tetroxide.
Nomenclature rules can also be used to determine the chemical formula based on the chemical name of the compound.
What is the chemical formula of carbon monosulphide?
The first word in the chemical name is carbon. Therefore, the chemical formula starts with 1 carbon atom |(\text{C}).|
The second word in the chemical name is monosulphide. It is composed of the prefix mono- and the root sulphide. Therefore, the chemical formula ends with 1 sulphur atom |(\text{S}).|
The chemical formula of carbon monosulphide is |\text{CS}.|
Note: The use of the prefix mono- indicates that other combinations are possible between carbon |(\text{C})| and sulfur |(\text{S}).|
What is the chemical formula of diphosphorus pentoxide?
The first word in the chemical name is diphosphorus. It is composed of the prefix di- and the root phosphorus. Therefore, the chemical formula starts with 2 phosphorus atoms |(\text{P}).|
The second word in the chemical name is pentoxide. It is composed of the prefix penta- and the root oxide. Therefore, the chemical formula ends with 5 oxygen atoms |(\text{O}).|
The chemical formula of diphosphorus pentoxide is |\text{P}_2 \text{O}_5.|
Ionic binary compounds are made up of a metal and a nonmetal. Ionic bonds are formed due to the transfer of electrons between the atoms.
The periodic table can be used to identify metals and nonmetals. The following nomenclature rules can be used to name ionic binary compounds.
-
Start by naming the atom that appears first in the chemical formula (usually a metal) using the element name in the periodic table.
-
Name the second atom (usually a nonmetal) by altering its element name and adding the suffix -ide to the root, as shown in the reference table.
Prefixes are not used when naming ionic compounds. The rules of chemical notation are used to indicate the number of atoms of each element in the compound.
Use the nomenclature rules to name the compound with the following chemical formula: |\text{CaCl}_2.|
The ionic compound |\text{CaCl}_2| is made up of 1 calcium atom |\text{Ca}| and 2 chlorine atoms |\text{Cl}|.
-
Since |\text{Ca}| is a metal, it should be named first using its element name in the periodic table: calcium.
-
The element name of the nonmetal atom |\text{Cl}| has to be converted using the suffix -ide: it becomes chloride. Prefixes are not used in ionic compounds.
The name of the compound with the chemical formula |\text{CaCl}_2| is calcium chloride.
Use the nomenclature rules to name the compound with the following chemical formula: |\text{Al}_2 \text{O}_3.|
The ionic compound |\text{Al}_2 \text{O}_3| is made up of 2 aluminum atoms |\text{Al}| and 3 oxygen atoms |\text{O}|.
-
Since |\text{Al}| is a metal, it should be named first using its element name in the periodic table: aluminum.
-
The element name of the nonmetal atom |\text{O}| has to be converted using the suffix -ide: it becomes oxide. Prefixes are not used in ionic compounds.
The name of the compound with the chemical formula |\text{Al}_2 \text{O}_3| is aluminum oxide.
The suffix -ide indicates a negative ionic charge (and/or a negative oxidation state).
For example, the |\text{NaCl}| compound is made up of a |\text{Na}| atom and a |\text{Cl}| atom that appear as |\text{Na}^+| and |\text{Cl}^{-}| ions. The negative |\text{Cl}^{-}| ion is referred to as chloride. The name of the |\text{NaCl}| compound is sodium chloride.
On the other hand, chlorine gas |\text{Cl}_2| is made up of two neutral chlorine atoms. Therefore, the suffix -ide does not apply. The |\text{Cl}_2| compound is called molecular chlorine.
Nomenclature rules can also be used to determine the chemical formula based on the chemical name of the compound.
What is the chemical formula of potassium iodide?
The first word in the chemical name points to the metal potassium. Therefore, the chemical formula starts with potassium |(\text{K}).|
The second word in the chemical name points to the nonmetal iodide. It shares the root with iodine. Therefore, the chemical formula ends with iodine |(\text{I}).|
The rules of chemical notation can be used to determine how many times each element appears in the compound. The chemical formula of potassium iodide is |\text{KI}.|
A polyatomic ion is an ion composed of several atoms.
The following reference table contains common polyatomic ions. They are named using advanced nomenclature rules. It would be helpful to memorize the names of the ions.
| Chemical formula | Name | Chemical formula | Name |
|---|---|---|---|
| |\text{CH}_3 \text{COO}^-| | Acetate | |\text{CN}^-| | Cyanide |
| |{\text{CO}_3}^{2-}| | Carbonate | |{\text{NH}_{4}}^+| | Ammonium |
| |{\text{HCO}_3}^{-}| | Bicarbonate | |{\text{NO}_2}^-| | Nitrite |
| |\text{ClO}^-| | Hypochlorite | |{\text{NO}_3}^-| | Nitrate |
| |{\text{ClO}_2}^-| | Chlorite | |\text{OH}^-| | Hydroxide |
| |{\text{ClO}_3}^-| | Chlorate | |{\text{PO}_4}^{3-}| | Phosphate |
| |{\text{ClO}_4}^-| | Perchlorate | |{\text{SO}_3}^{2-}| | Sulphite |
| |{\text{CrO}_4}^{2-}| | Chromate | |{\text{SO}_4}^{2-}| | Sulphate |
Polyatomic ions often bond with other atoms to form ionic compounds. In this case, the nomenclature rules for naming compounds with polyatomic ions are very similar to the rules for naming ionic binary compounds presented in the previous section.
-
Start by naming the atom or the group of atoms that appear first in the chemical formula.
-
If the compound’s chemical formula begins with an atom (usually a metal), name it using its element name in the periodic table.
-
If the compound’s chemical formula begins with a polyatomic ion, name it using the reference table in the previous section.
-
-
Name the atom or the group of atoms that appears second.
-
If the compound’s chemical formula ends with a polyatomic ion, name it using the reference table in the previous section.
-
If the compound’s chemical formula ends with an atom (usually a nonmetal), name it by altering its element name and adding the suffix -ide to the root, as shown in the reference table.
-
Prefixes are not used when naming ionic compounds with polyatomic ions. Instead, the rules of chemical notation are used to indicate the number of each ion in the compound.
Use the nomenclature rules to name the compound with the following chemical formula: |\text{KClO}_3.|
Using the table of polyatomic ions, the |\text{KClO}_3| compound can be split into the |\text{K}| atom and the |\text{ClO}_3| group of atoms.
-
Since the |\text{K}| atom appears first, it should be named first using its element name in the periodic table: potassium.
-
The |\text{ClO}_3| group corresponds to the |{\text{ClO}_3}^-| polyatomic ion. According to the reference table, it is called chlorate.
The name of the compound with the chemical formula |\text{KClO}_3| is potassium chlorate.
Use the nomenclature rules to name the compound with the following chemical formula: |\text{Mg(NO}_3 \text{)}_2.|
Using the table of polyatomic ions, the |\text{Mg(NO}_3 \text{)}_2| compound can be split into the |\text{Mg}| atom and the |\text{NO}_3| group of atoms, which appears twice, as indicated by the subscript |2.|
-
Since the |\text{Mg}| atom appears first, it should be named first using its element name in the periodic table: magnesium.
-
The |\text{NO}_3| group corresponds to the |{\text{NO}_3}^-| polyatomic ion. According to the reference table it is called nitrate. Prefixes are not used in ionic compounds.
The name of the compound with the chemical formula |\text{Mg(NO}_3 \text{)}_2| is magnesium nitrate.
Use the nomenclature rules to name the compound with the following chemical formula: |\text{NH}_4 \text{F}.|
Using the table of polyatomic ions, the |\text{NH}_4 \text{F}| compound can be split into the |\text{NH}_4| group of atoms and the |\text{F}| atom.
-
The |\text{NH}_4| group appears first and corresponds to the |{\text{NH}_4}^+| polyatomic ion. According to the reference table it is called ammonium.
-
The element name of the second atom |\text{F}| has to be converted using the suffix -ide: it becomes fluoride.
The name of the compound with the chemical formula |\text{NH}_4 \text{F}| is ammonium fluoride.
Nomenclature rules can also be used to determine the chemical formula based on the chemical name of the compound.
What is the chemical formula of sodium nitrate?
The first word in the chemical name indicates the metal sodium. Therefore, the chemical formula starts with a sodium atom |(\text{Na}).|
The second word in the chemical name indicates the polyatomic ion nitrate. According to the reference table, it is the |{\text{NO}_3}^-| ion. It will appear in the chemical formula as |\text{NO}_3.|
The rules of chemical notation can be used to determine how many times each ion appears in the compound. The chemical formula of sodium nitrate is |\text{Na} \text{NO}_3.|
In addition to the elements, covalent and ionic binary compounds, and compounds with polyatomic ions, other substances are sometimes used in textbooks. Notably, certain organic compounds and complex inorganic compounds can be referenced.
Naming these substances requires additional nomenclature rules that are not covered in secondary school.
The following table includes the chemical formulas and names of some of the substances.
| Chemical formula | Name |
|---|---|
| |\text{CH}_4| | Methane |
| |\text{CH}_3 \text{CH}_2 \text{CH}_3| or |\text{C}_3 \text{H}_8| | Propane |
| |\text{CH}_3 \text{CH}_2 \text{CH}_2 \text{CH}_3| or |\text{C}_4 \text{H}_{10}| | Butane |
| |\text{CH}_3 \text{COOH}| | Acetic acid |
| |\text{C}_6 \text{H}_{12} \text{O}_6| | Glucose |
| |\text{NH}_3| | Ammonia |
| |\text{O}_3| | Ozone |