When two atoms interact, they can form a chemical bond. Usually, this chemical bond is covalent or ionic.
|
Covalent bond |
Ionic bond |
|
|---|---|---|
|
Bound elements |
Non-metal bound to a non-metal |
Non-metal bound to a metal (with some exceptions) |
|
Interaction between bound elements |
Shared electrons |
Transferred electron(s) |
|
Electronegativity difference between bound atoms |
Low to medium |
High |
|
Ion formation |
Ions do not form |
Ions form |
|
Type of substance formed |
|
|
Note: Metalloids are elements with the ability to make covalent or ionic bonds, depending on the context. Therefore, they are not included in this table.
A chemical bond between two atoms is the result of the sharing or transfer of electron(s) between these two atoms.
A bond usually makes the two atoms involved more stable by obtaining the same electron configuration as a noble gas. A noble gas is very stable, because its last electron shell contains the maximum number of electrons allowed. Its last electron shell is said to be full.
When an atom has the same electron configuration as a noble gas, it is said to follow the duet or octet rule. This gives the atom great stability.
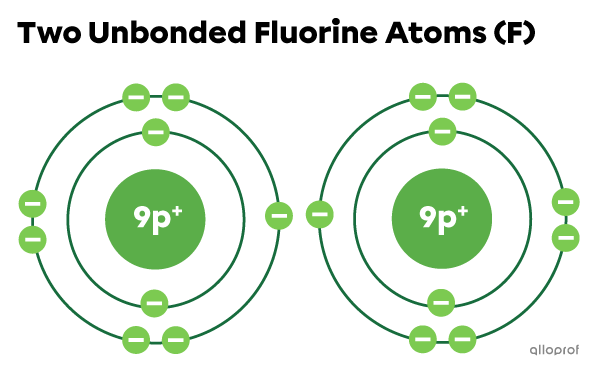
When the two fluorine |(\text{F})| atoms are not bonded, they each have 7 electrons on the electron shell furthest from the nucleus. Therefore, they do not have the electron configuration of a noble gas.
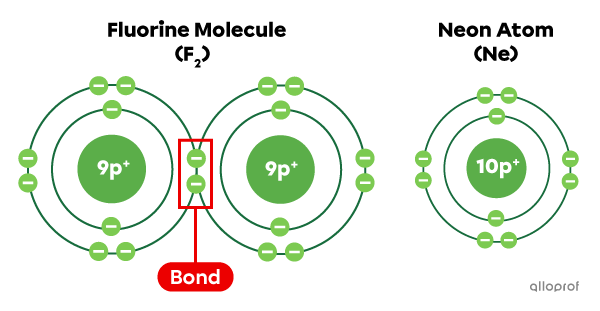
When the two fluorine |(\text{F})| atoms bond, they form a fluorine molecule |\text{F}_2.| By sharing electrons, each fluorine atom has 8 electrons on its last electron shell, which corresponds to the electron configuration of neon |(\text{Ne}).| This makes both fluorine atoms |(\text{F})| more stable.
There are several types of chemical bonds. The most common bonds are covalent bonds and ionic bonds.
A covalent bond is the sharing of an electron pair between two atoms. These atoms are usually non-metals.
The periodic table of elements can be used to identify non-metals.
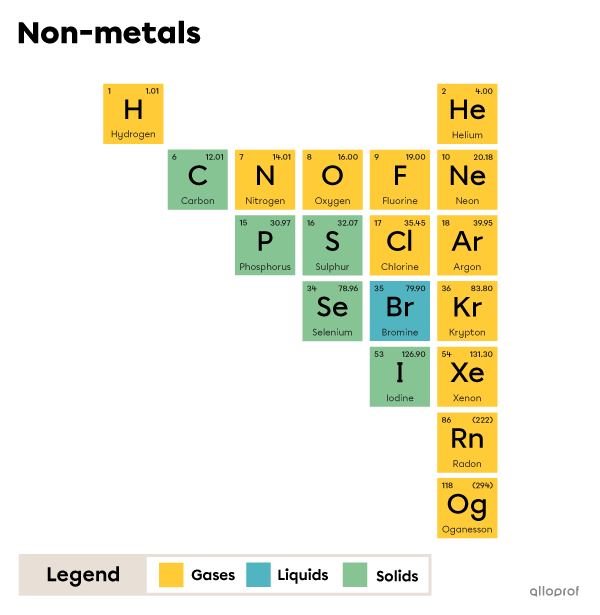
Metalloids are elements that have properties of both metals and non-metals. This means that metalloids have the ability to form covalent or ionic bonds.
Boron |(\text{B})| is a metalloid.
In borane |(\text{BH}_3),| the boron atom |(\text{B})| behaves like a non-metal. In this case, the atom of boron |(\text{B})| forms a covalent bond with each atom of hydrogen |(\text{H}).|
In boron trifluoride |(\text{BF}_3),| the boron atom |(\text{B})| behaves like a metal. In this case, the atom of boron |(\text{B})| forms an ionic bond with each atom of fluorine |(\text{F}).|
Every atom is characterized by its specific electronegativity.
Electronegativity is the force with which the nucleus of an atom attracts the electrons involved in a chemical bond.
The electronegativity difference between two non-metals involved in a covalent bond is usually low to medium. This makes electron sharing between the two non-metals relatively balanced. In other words, neither atom involved in a covalent bond “pulls” on the electrons strongly enough to result in their permanent transfer.
A group of atoms with only covalent bonds is a molecule. If this molecule contains different atoms, it can also be called a covalent compound or a molecular compound.
There are several ways to represent a covalent bond. For example, the Rutherford-Bohr model or Lewis notation can be used.
Here, the formation of the hydrofluoric acid molecule |(\text{HF})| is represented using the Rutherford-Bohr model as well as Lewis notation. In this molecular compound, the bond between the hydrogen atom |(\text{H})| and the fluorine atom |(\text{F})| is covalent.
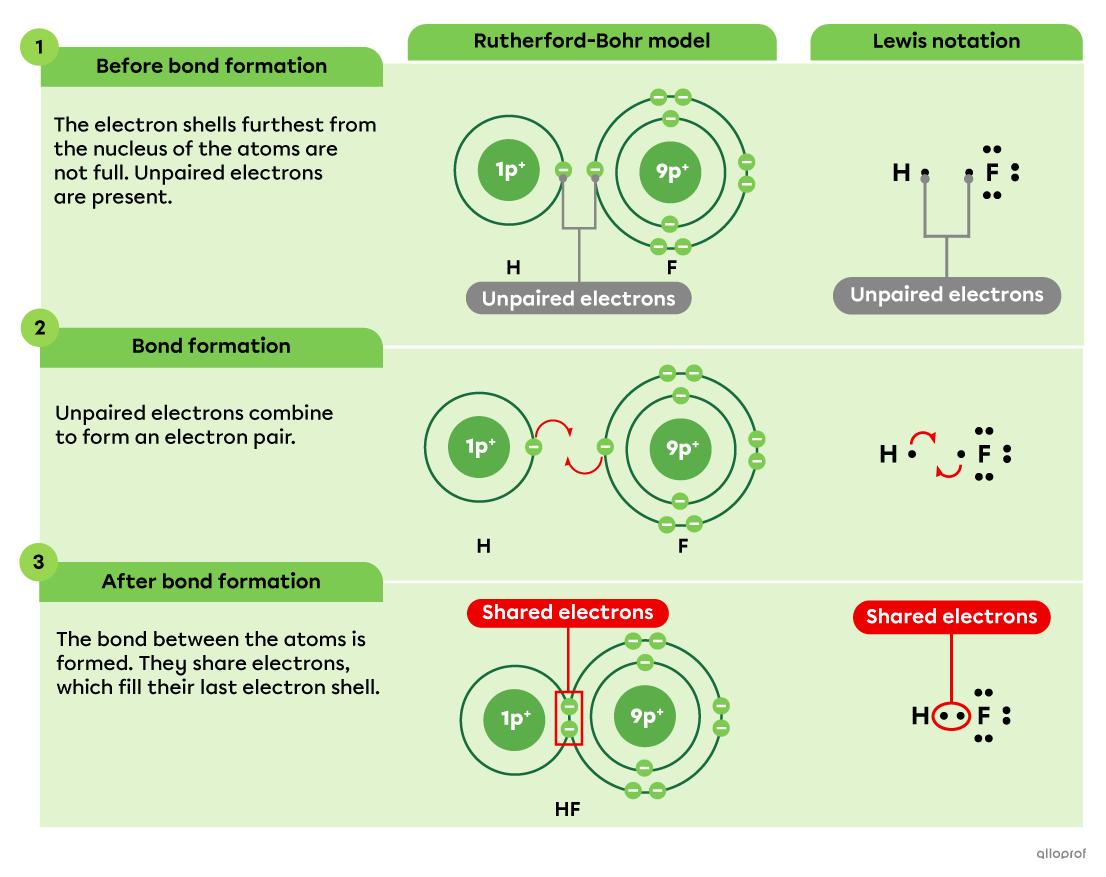
While the Rutherford-Bohr model shows all the electrons present in the molecule, Lewis notation only shows the valence electrons, which are the electrons in the electron shell furthest from the nucleus.
Moreover, when a covalent bond between two atoms is formed, both representations show that 2 electrons are shared between the atoms. This pair of electrons is the bonding pair responsible for the covalent bond.
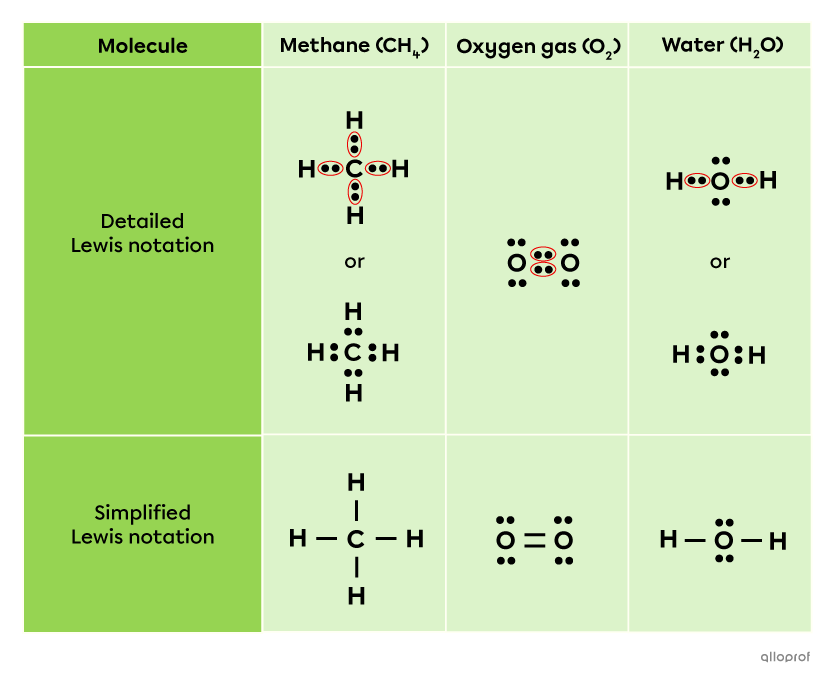
When the bond is represented using detailed Lewis notation, the bonding pair is shown by circling the two shared electrons. This diagram can be simplified by replacing the circled dots with a short line. Every lone pair of electrons, which is a pair not involved in bond formation, is always represented by two dots next to each other.
The following table shows the structure of several molecules using detailed Lewis notation and simplified Lewis notation.
Two atoms can be linked by a single bond, a double bond, a triple bond or, more rarely, a quadruple bond.
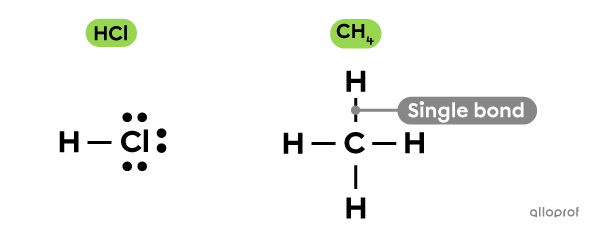
When two atoms share 1 electron pair (2 electrons), a single bond is formed. This occurs between atoms in a methane molecule |(\text{CH}_4)| or a hydrochloric acid molecule |(\text{HCl}).|
One line is used to represent a single bond.
When two atoms share 2 electron pairs (4 electrons), a double bond is formed. This occurs between atoms in an oxygen molecule |(\text{O}_2)| or a carbon dioxide molecule |(\text{CO}_2).|
Two lines are used to represent a double bond.

When two atoms share 3 electron pairs (6 electrons), a triple bond is formed. This occurs between atoms in a nitrogen molecule |(\text{N}_2)| or between carbon |(\text{C})| and nitrogen |(\text{N})| atoms in a hydrogen cyanide molecule |(\text{HCN}).|
Three lines are used to represent a triple bond.

Although rarely, sometimes two atoms can share 4 electron pairs (8 electrons in total) to form a quadruple bond. This occurs with certain transition metals (metals from groups 3 to 12), such as ruthenium |(\text{Ru}).|
An ionic bond is the transfer of electron(s) which usually occurs between a metal and a non-metal.
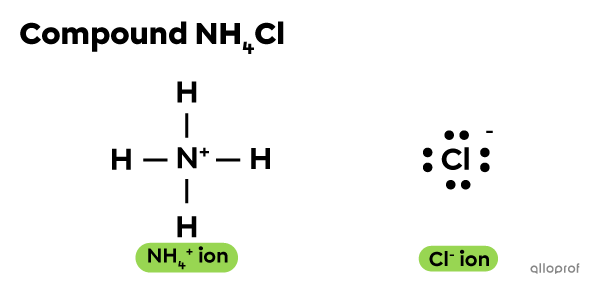
Sometimes, an ionic bond forms when a polyatomic ion such as ammonium |({\text{NH}_{4}}^+)| interacts with another ion.
For example, ammonium chloride |(\text{NH}_4 \text{Cl})| is a compound that consists of an ammonium |({\text{NH}_{4}}^+)| ion and a chloride ion |(\text{Cl}^{-}).| Neither of these ions contains metal atoms. And yet, the |({\text{NH}_{4}}^+)| ion and the |\text{Cl}^-| ion form an ionic bond.
In some instances, metalloids can form ionic bonds as well. Since the case of metalloids is more complex, it will not be discussed in this concept sheet.
The periodic table of elements can be used to identify metals and non-metals.
Every atom is characterized by its specific electronegativity. The electronegativity difference between a metal and a non-metal involved in an ionic bond is usually high. This means that the electrons involved in the bond are not evenly distributed between the two atoms: the electrons are strongly attracted to the more electronegative atom.
The non-metal atom "pulls" one or more electrons away from the metal atom. Thus, the non-metal has a surplus of electrons and becomes negative. A negative ion is called an anion.
The metal atom, which loses one or more electrons, becomes positive. A positive ion is called a cation.
The following table summarizes the behaviour of the atoms involved in ionic bond formation.
|
Metal |
Non-metal |
|
|---|---|---|
|
Electronegativity |
Lower |
Higher |
|
Tends to |
Lose electron(s) |
Gain electron(s) |
|
Ion formed |
Cation (positive ion) |
Anion (negative ion) |
A compound containing an ionic bond is made up of ions. When such a compound is placed in an aqueous solution, the released ions enable the flow of electric current. Therefore, ionic compounds are electrolytic substances.
A compound with one or more ionic bonds is not a molecule, but a salt, also referred to as an ionic compound.
Some compounds contain both ionic and covalent bonds. Since they contain at least one ionic bond, they are still considered to be ionic compounds.
There are several ways to represent an ionic bond. For example, the Rutherford-Bohr model or Lewis notation can be used.
Here, the formation of lithium fluoride |(\text{LiF})| is represented using the Rutherford-Bohr model as well as Lewis notation. In this compound, the bond between the lithium atom |(\text{Li})| and the fluorine atom |(\text{F})| is ionic.
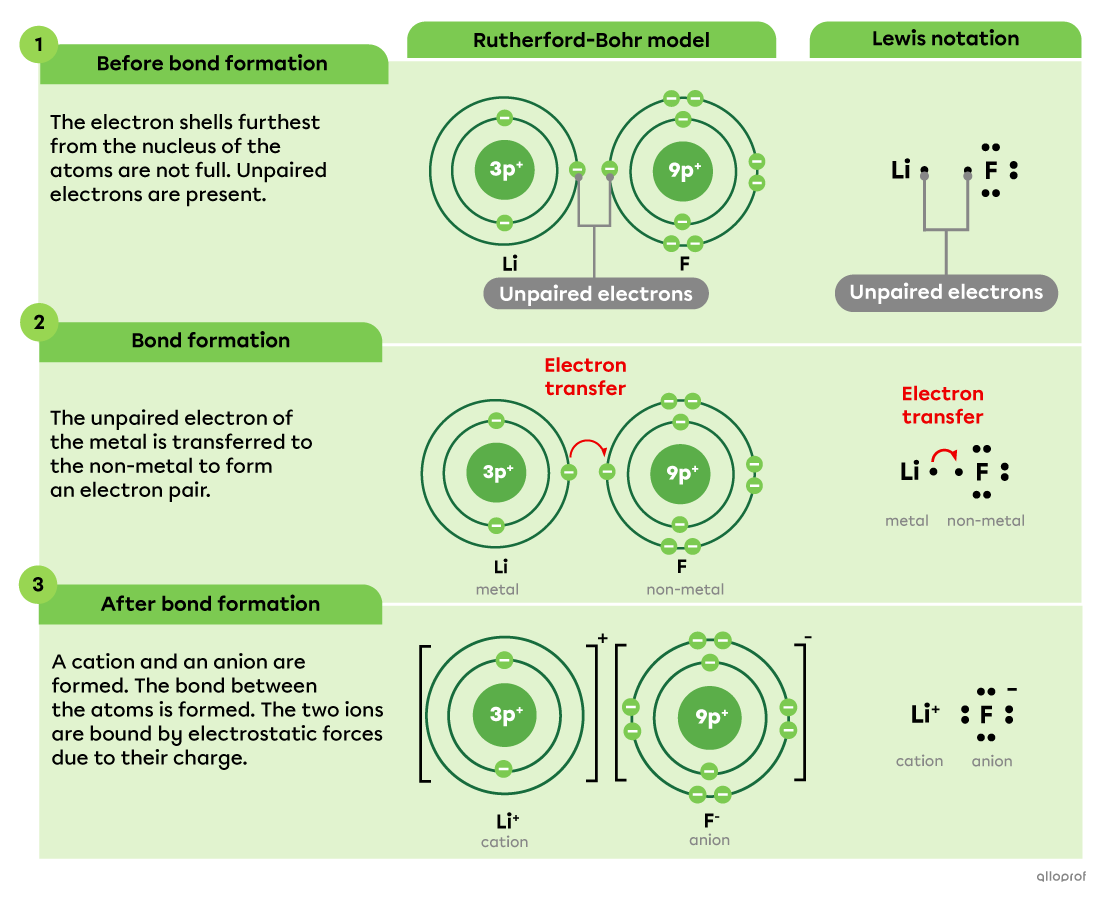
No matter which model is used, the following facts can be stated.
-
An electron is transferred from lithium |(\text{Li})| to fluorine |(\text{F}).| Due to its low electronegativity, lithium loses its valence electron to fluorine, which is highly electronegative.
-
By losing an electron, the lithium atom becomes a cation |(\text{Li}^+)| and acquires the electron configuration of the noble gas helium |(\text{He}).|
-
By gaining an electron, the fluorine atom becomes a anion |(\text{F}^-)| and acquires the electron configuration of the noble gas neon |(\text{Ne}).|
The following examples represent the formation of several ionic compounds using Lewis notation.
-
In order to form sodium chloride |(\text{NaCl})| from a sodium atom |(\text{Na})| and a chlorine atom |(\text{Cl}),| an electron transfer occurs.
Sodium |(\text{Na}),| which is a metal, is an electron donor. Chlorine |(\text{Cl}),| which is a non-metal, is an electron acceptor.
A bond forms between the sodium ion |(\text{Na}^+)| and the chloride ion |(\text{Cl}^-)| due to electrostatic attraction between them. These ions form an ionic compound.
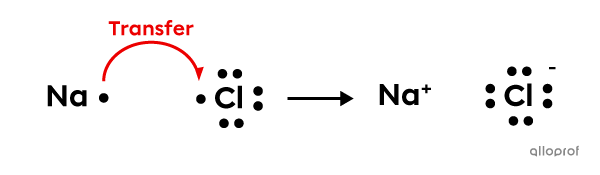
-
In order to form potassium oxide |(\text{K}_2 \text{O})| from two potassium atoms |(\text{K})| and one oxygen atom |(\text{O}),| 2 electron transfers occur.
Potassium |(\text{K}),| which is a metal, is an electron donor. Oxygen |(\text{O}),| which is a non-metal, is an electron acceptor.
Since each potassium atom transfers a single electron to the oxygen atom, each potassium ion formed has a single positive charge |(\text{K}^+).| The oxygen atom, which accepts a total of 2 electrons, becomes an oxide ion with two negative charges |(\text{O}^{2-}).|
The formed compound |(\text{K}_2 \text{O})| contains 2 ionic bonds.

-
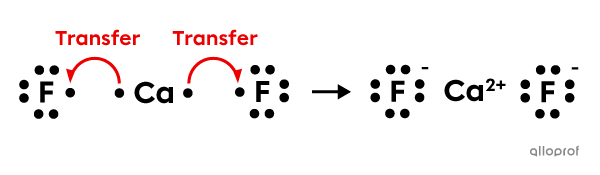
In order to form calcium fluoride |(\text{CaF}_2)| from one calcium atom |(\text{Ca})| and two fluorine atoms |(\text{F}),| 2 electron transfers occur.
Calcium |(\text{Ca}),| which is a metal, is an electron donor. Fluorine |(\text{F}),| which is a non-metal, is an electron acceptor.
Since the calcium atom gives up 2 electrons, it becomes a calcium ion with two positive charges |(\text{Ca}^{2+}).| Each fluorine atom accepts a single electron, which results in two negatively charged fluoride ions |(\text{F}^-).|
The formed compound |(\text{CaF}_2)| contains 2 ionic bonds.